Human fusion antibody for reducing cerebral amyloid fibers associated with senile dementia
A human antibody, amyloid peptide technology, applied in the direction of antibodies, drug combinations, introduction of foreign genetic material using vectors, etc., can solve problems such as the difficulty of anti-Aβ antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
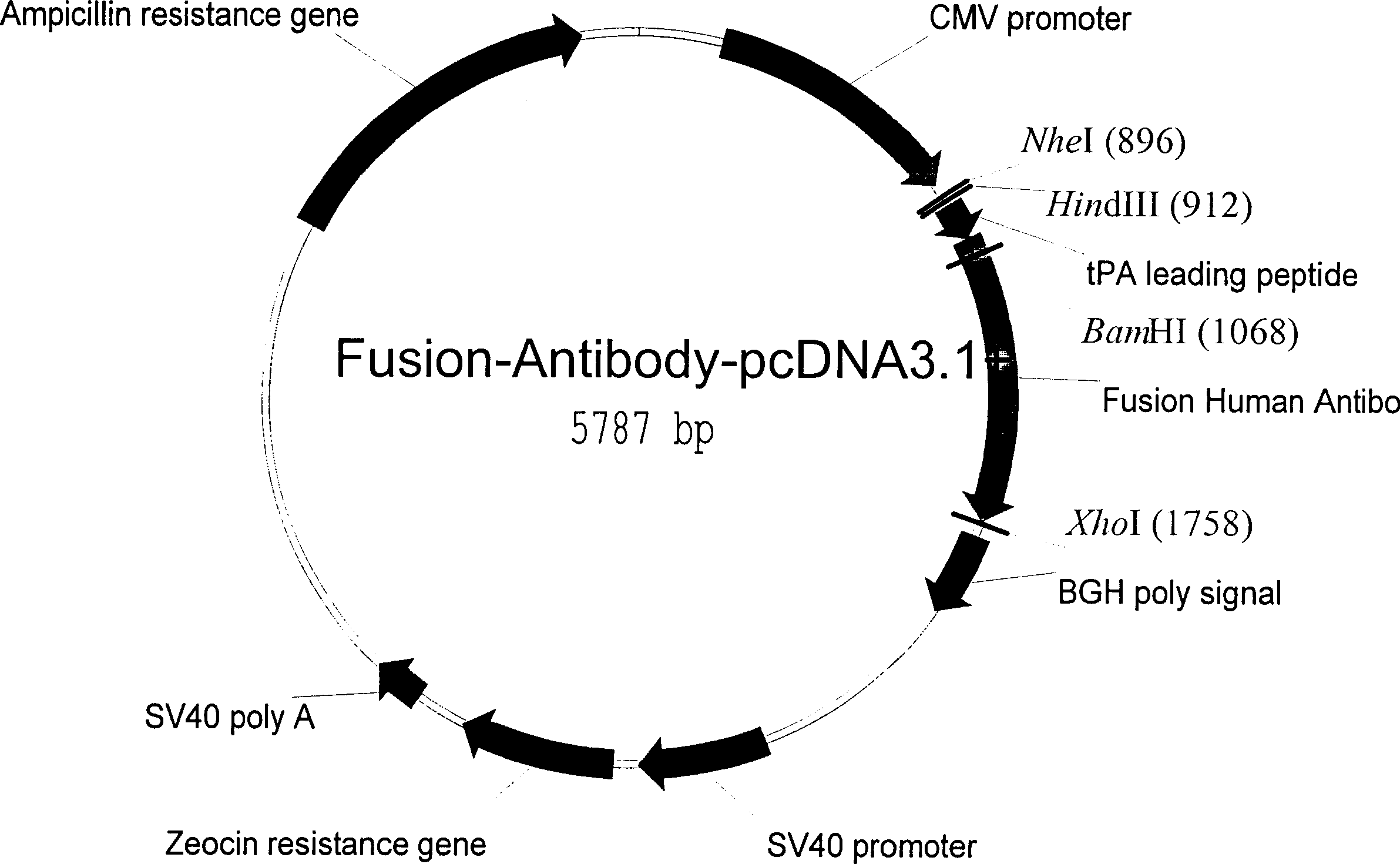
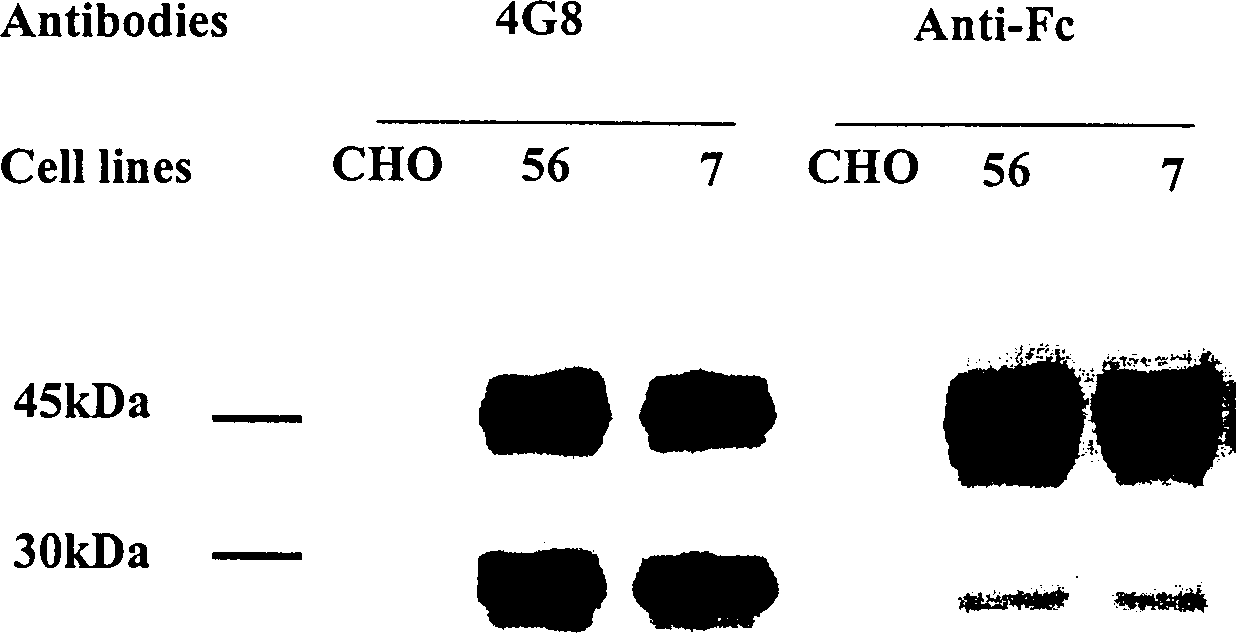
[0030] Example 1: Eukaryotic cell expression of fusion antibody
[0031] Construction of fusion human antibody DNA containing tPA signal polypeptide
[0032] Human antibody Fc genes were selectively amplified from a lymphocyte library using a PCR method. Choose EXPEND HIGH FIDELITY PCR KIT from ROCHE Company. The PCR reaction includes 38ul deionized water, 5ul 10X buffer provided by the kit, 3ul DMSO, 1ul dNTP (10mM dATP, 10mMdCTP, 10mM dGTP, 10mM dTTP), 1ul mixed DNA polymerase provided by the kit, 1ul10uM containing BamH1 endonuclease Enzyme site forward primer AGTTGGATCCGACAAAACTCACACATG and 1ul 10uM reverse primer TCTAGACTCGAGTTATTTACCCGGAGACAG containing XhoI endonuclease site, 1ul human lymphocyte cDNA library (CloneTech). The PCR reaction uses 94°C, 30 seconds; 54°C, 30 seconds; 72°C, 1 minute; a total of 30 cycles. After separation by 1% agar electrophoresis to confirm that the PCR product contains Fc-sized DNA, extract 1 ul and add 1 ul of 1uM concentration of the fol
Embodiment 2
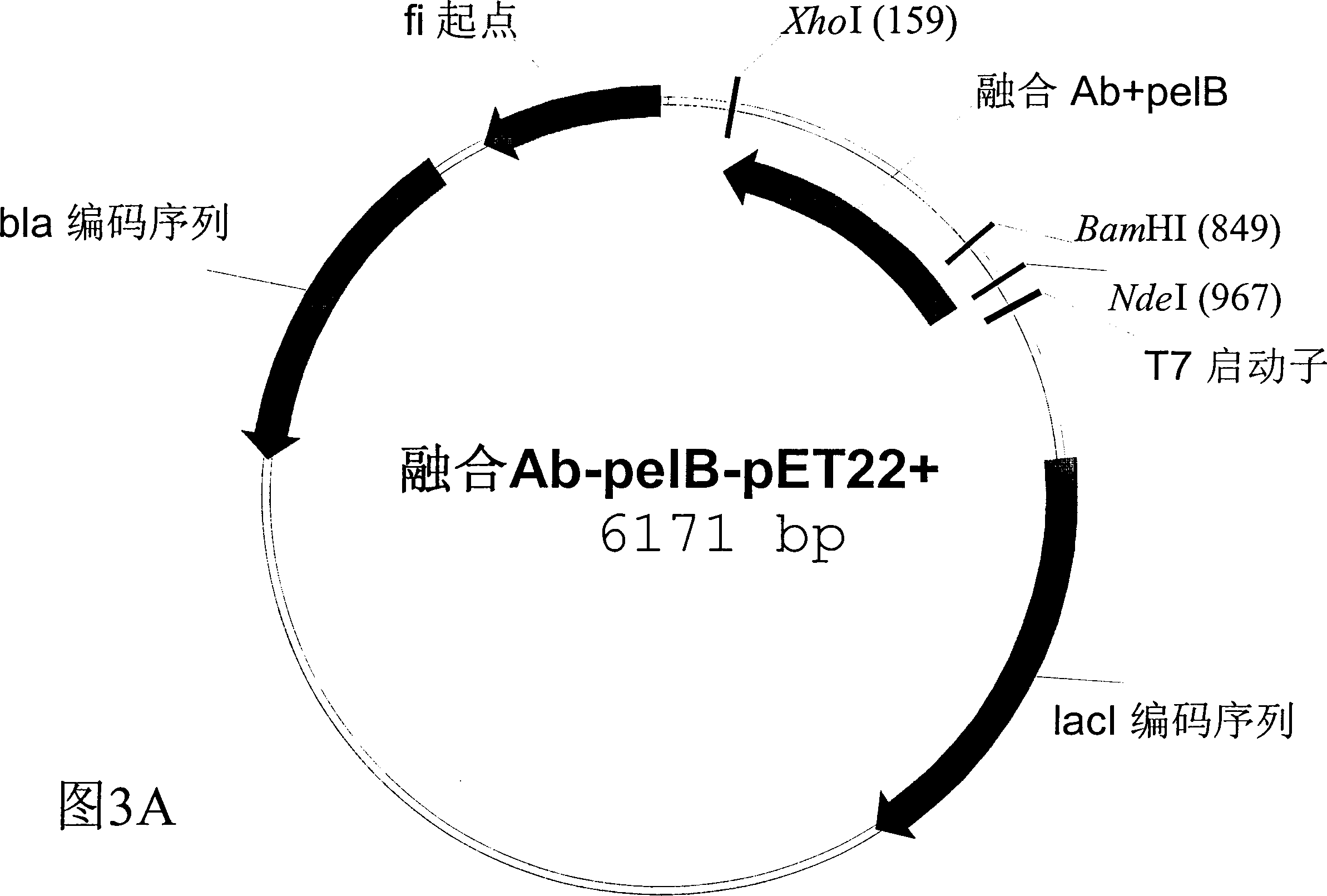
[0039] Example 2 Expression of Fusion Antibody in Bacterial Expression System
[0040] There are two types of fusion human antibodies expressed in bacteria. One is to add a methionine only before the fusion antibody, and the fusion antibody is mainly concentrated in the inclusion body in the bacteria after expression; the other has a signal polypeptide secreted into the bacterial cell interstitium at the amino terminal of the fusion antibody. The basic requirement for a bacterial expression vector is a bacterial expression promoter at the 5' end of the target gene. The expression vector used in the present invention is pET22b+ (Novagen), which has a T7 promoter. The signal polypeptide sequence used in the present invention is the pelB signal polypeptide:
[0041] MKYLLPTAAAGLLLLAAQPAMA
[0042] The amino acid sequence of the fusion antibody containing the bacterial interstitial signal polypeptide is:
[0043] MKYLLPTAAAGLLLLAAQPAMAKL VFFAEDVGSNKGA VGSDKTHTCPPCPAPELLGGPSVFLF
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap