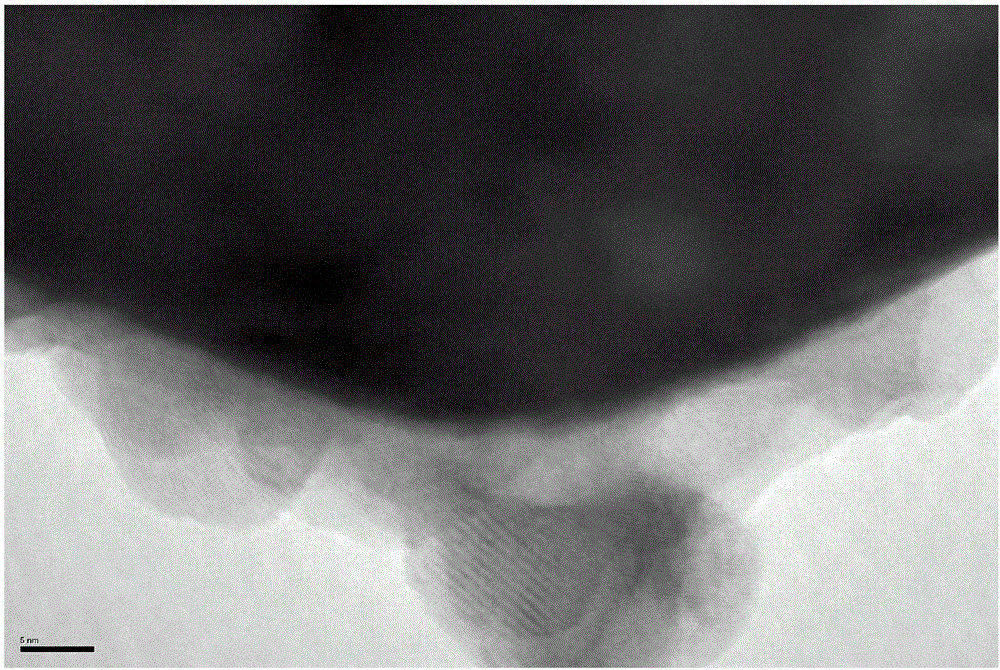
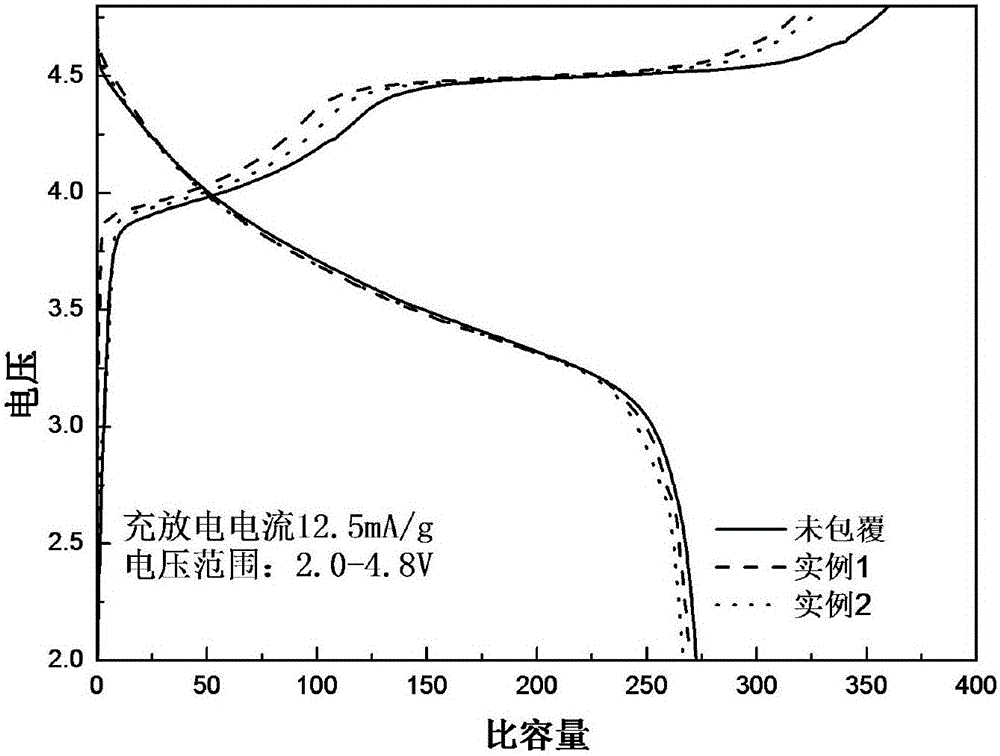
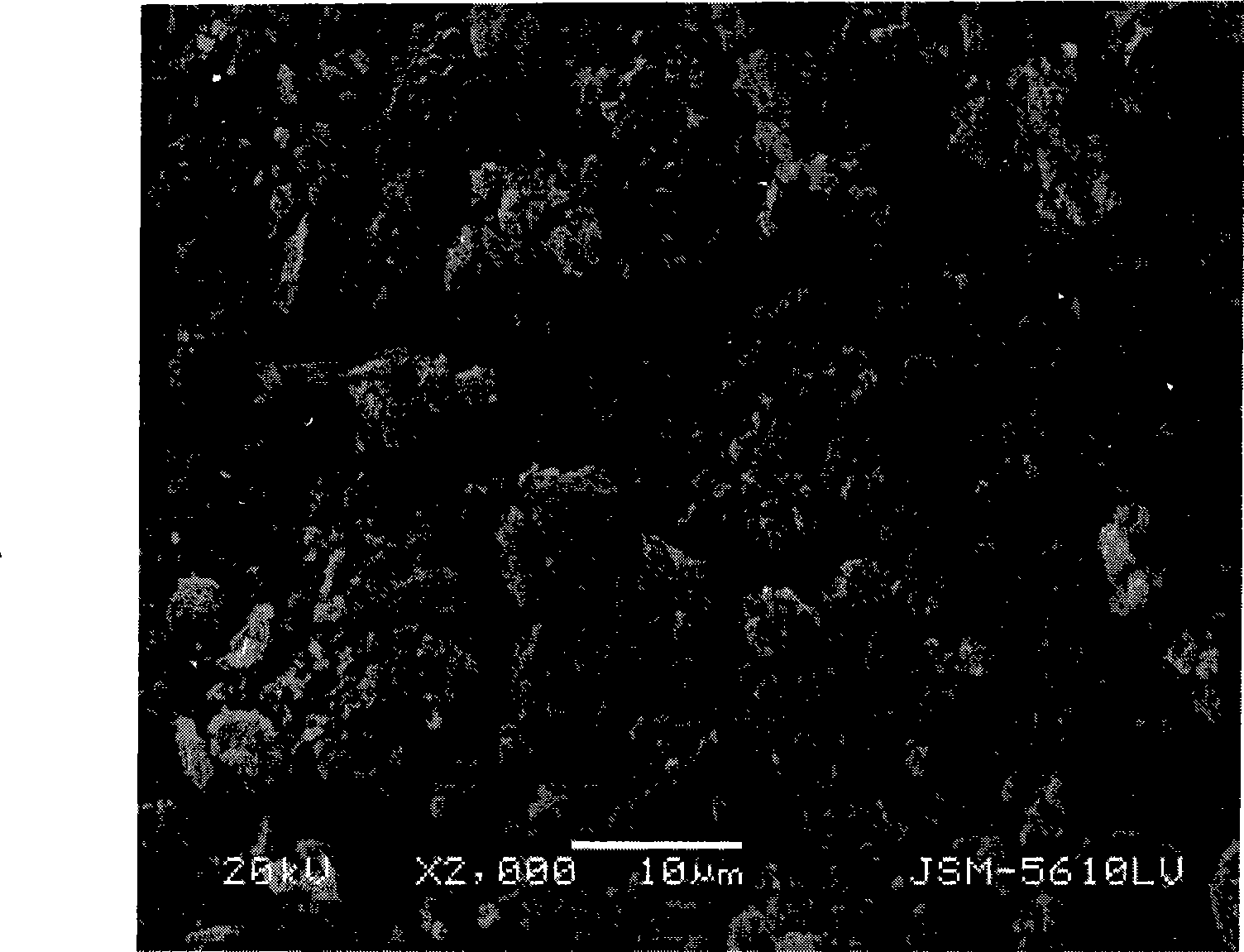
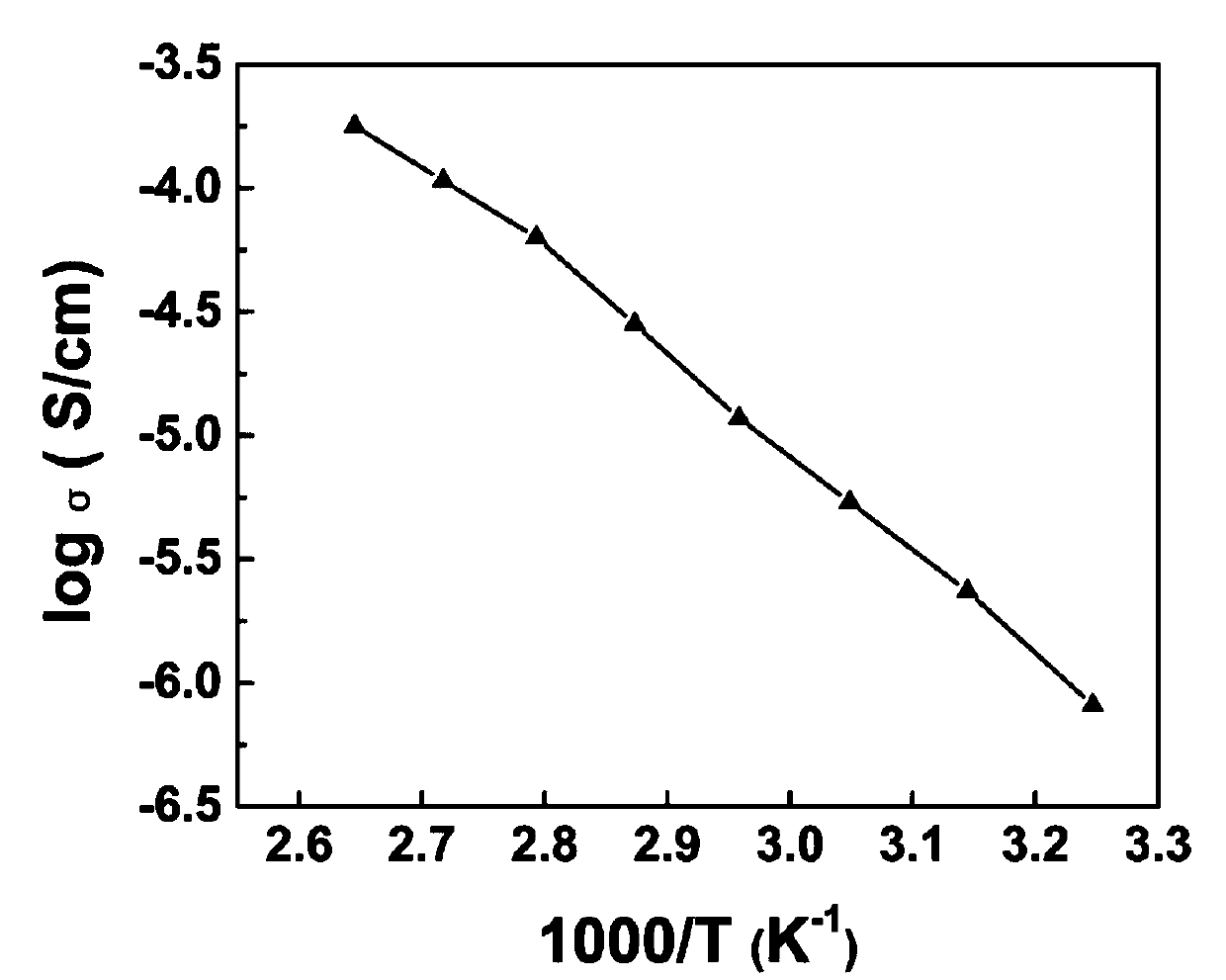
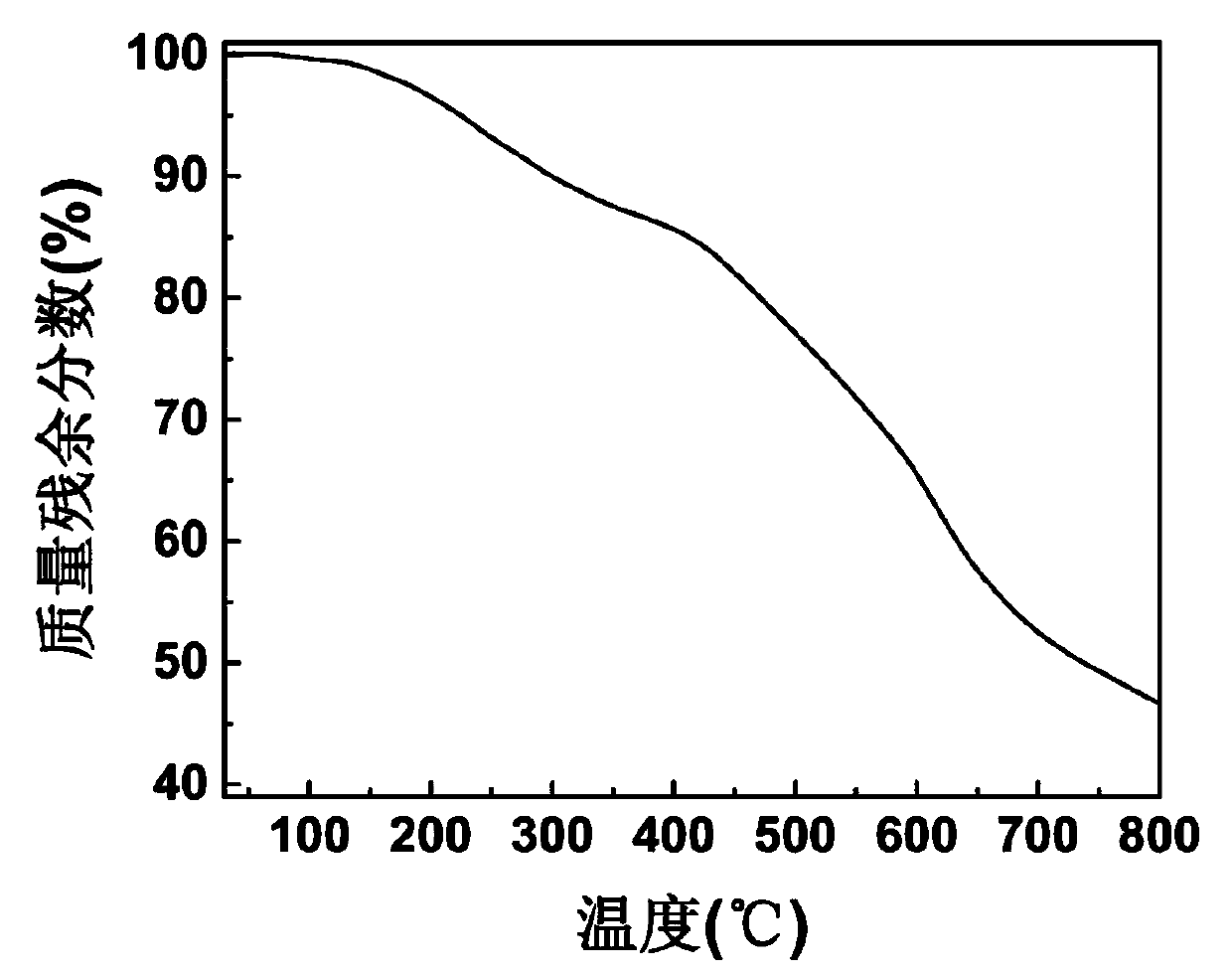
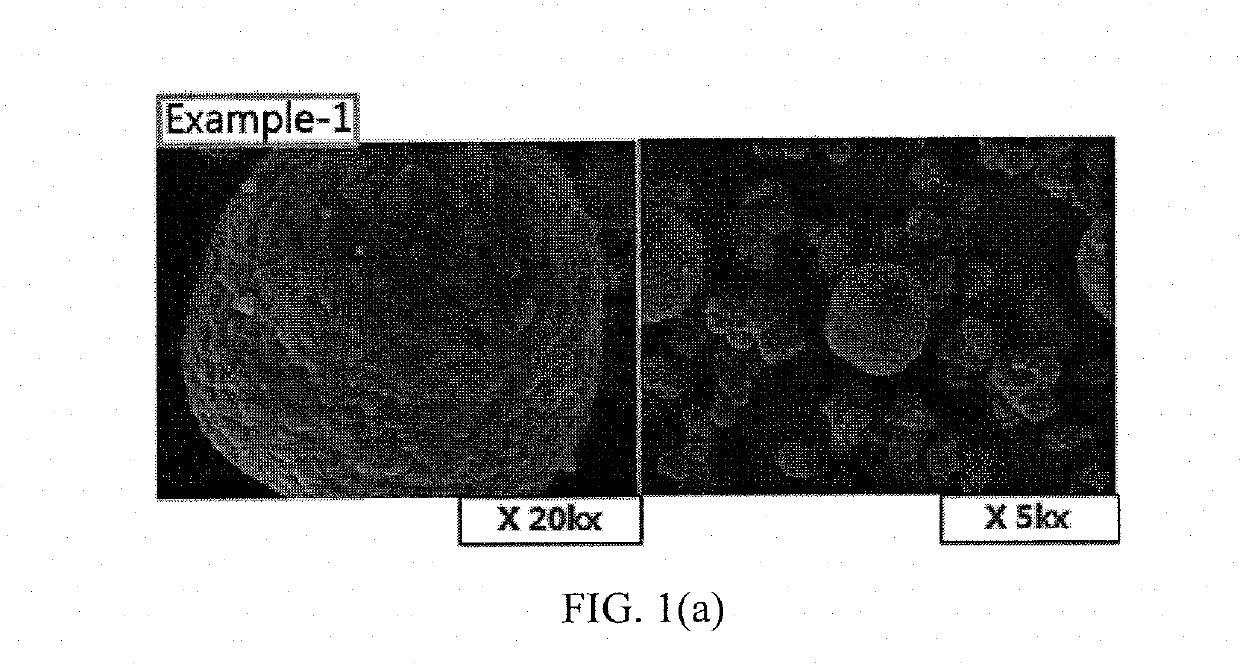
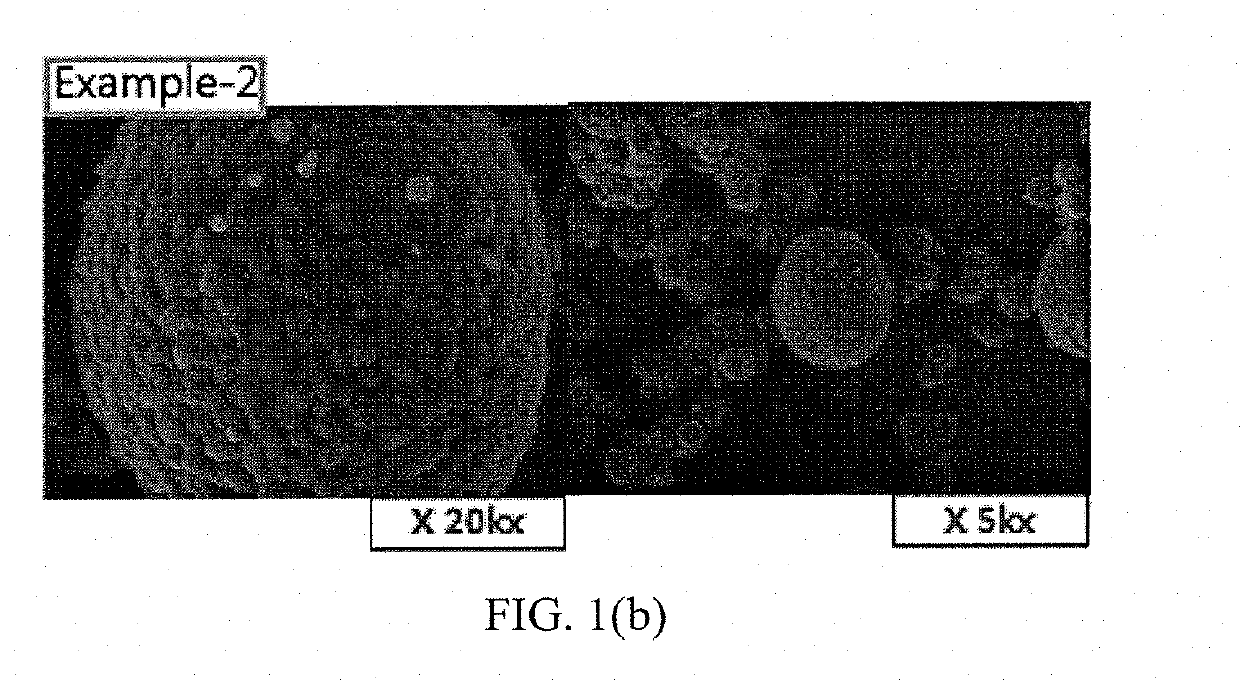
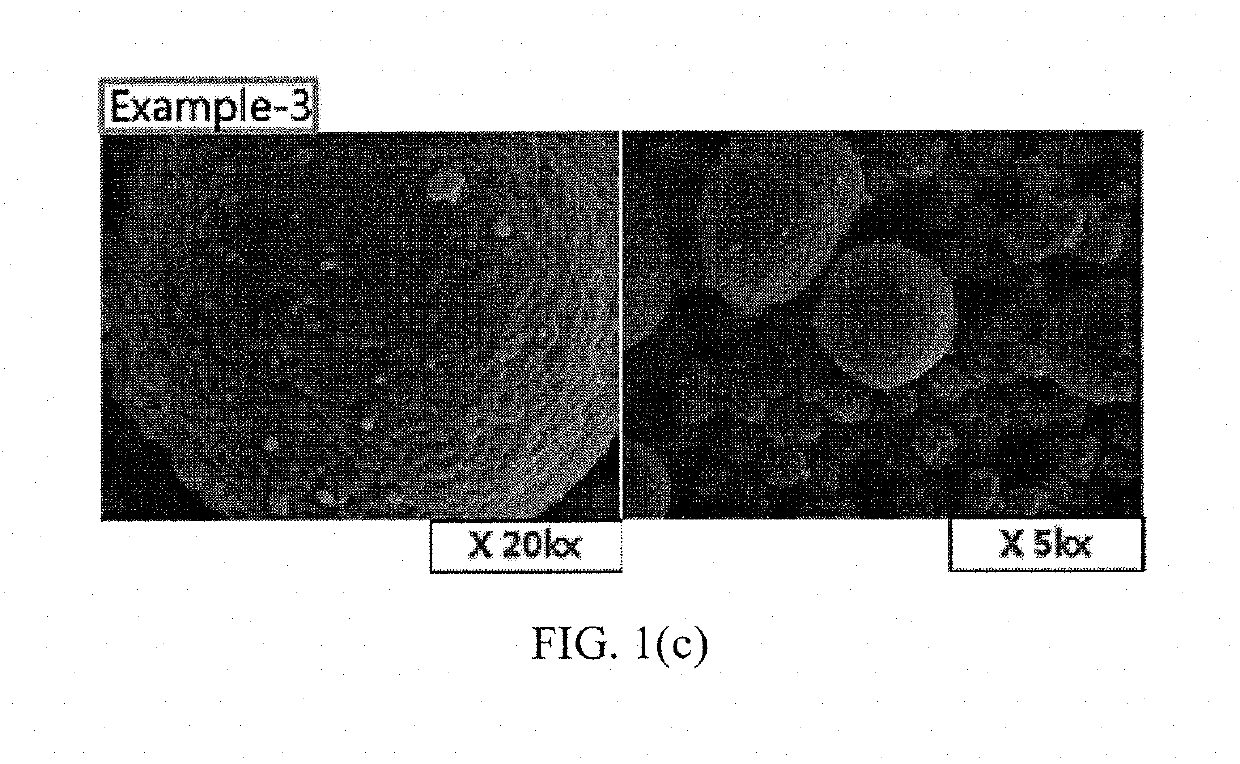
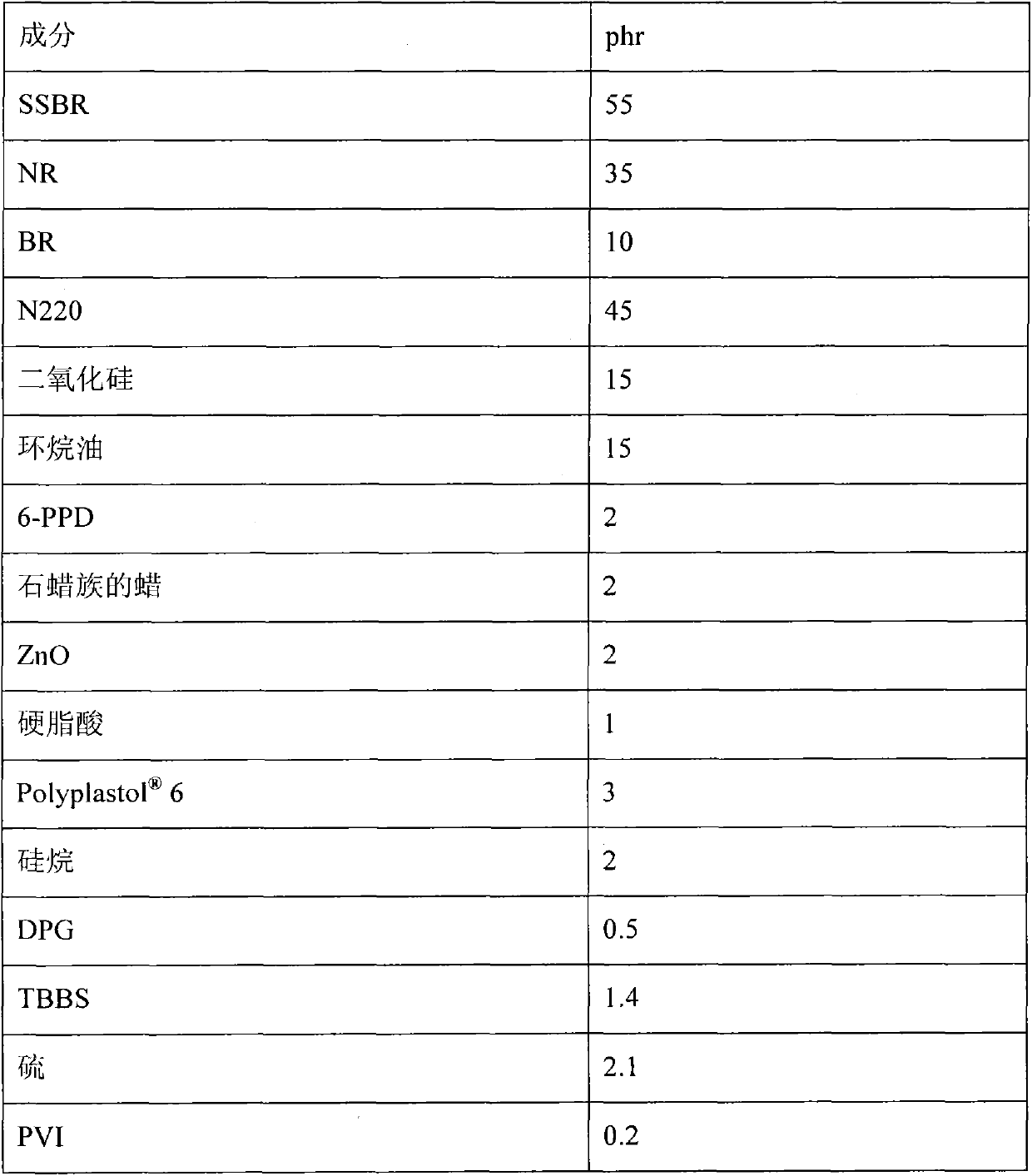
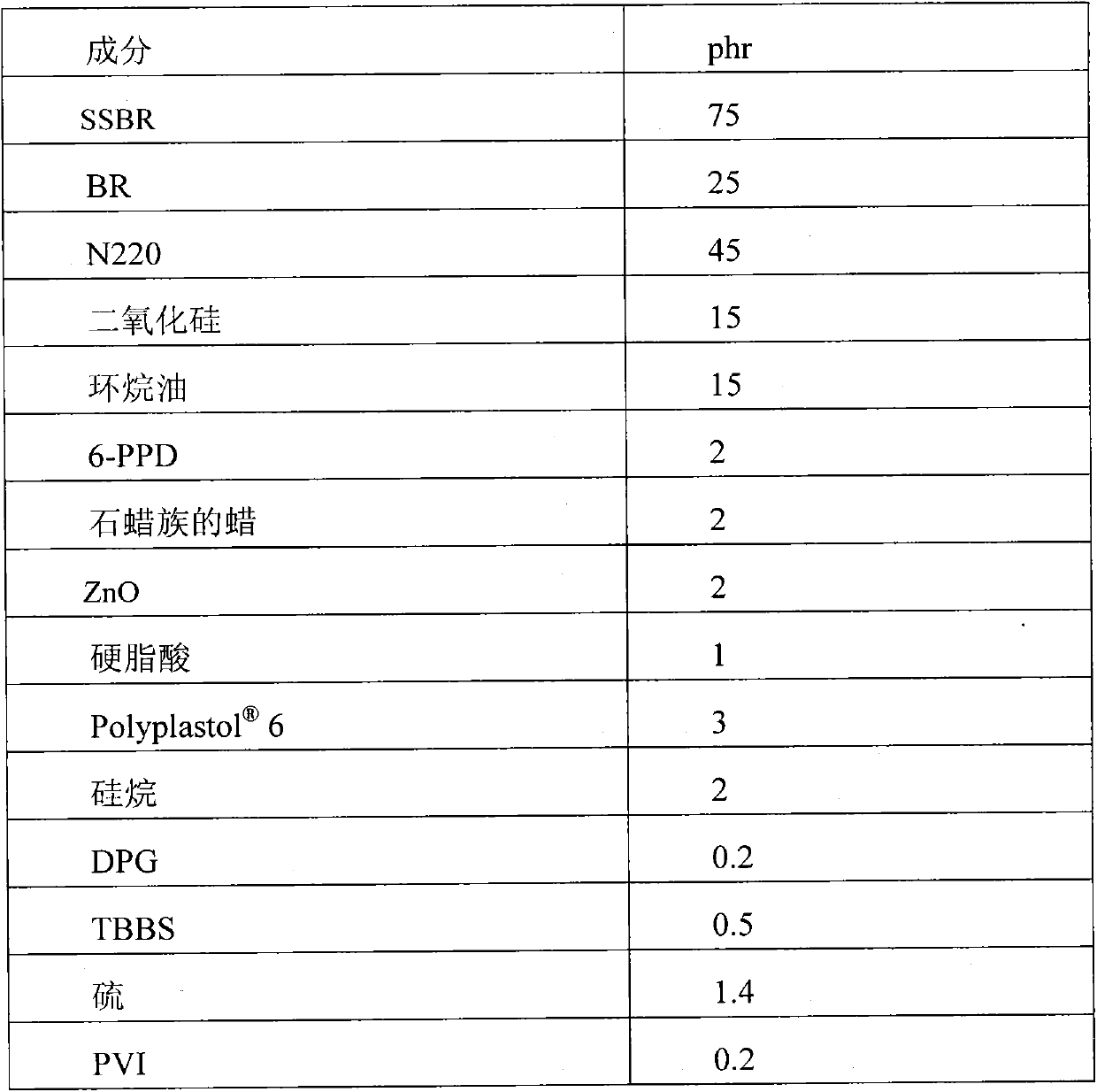
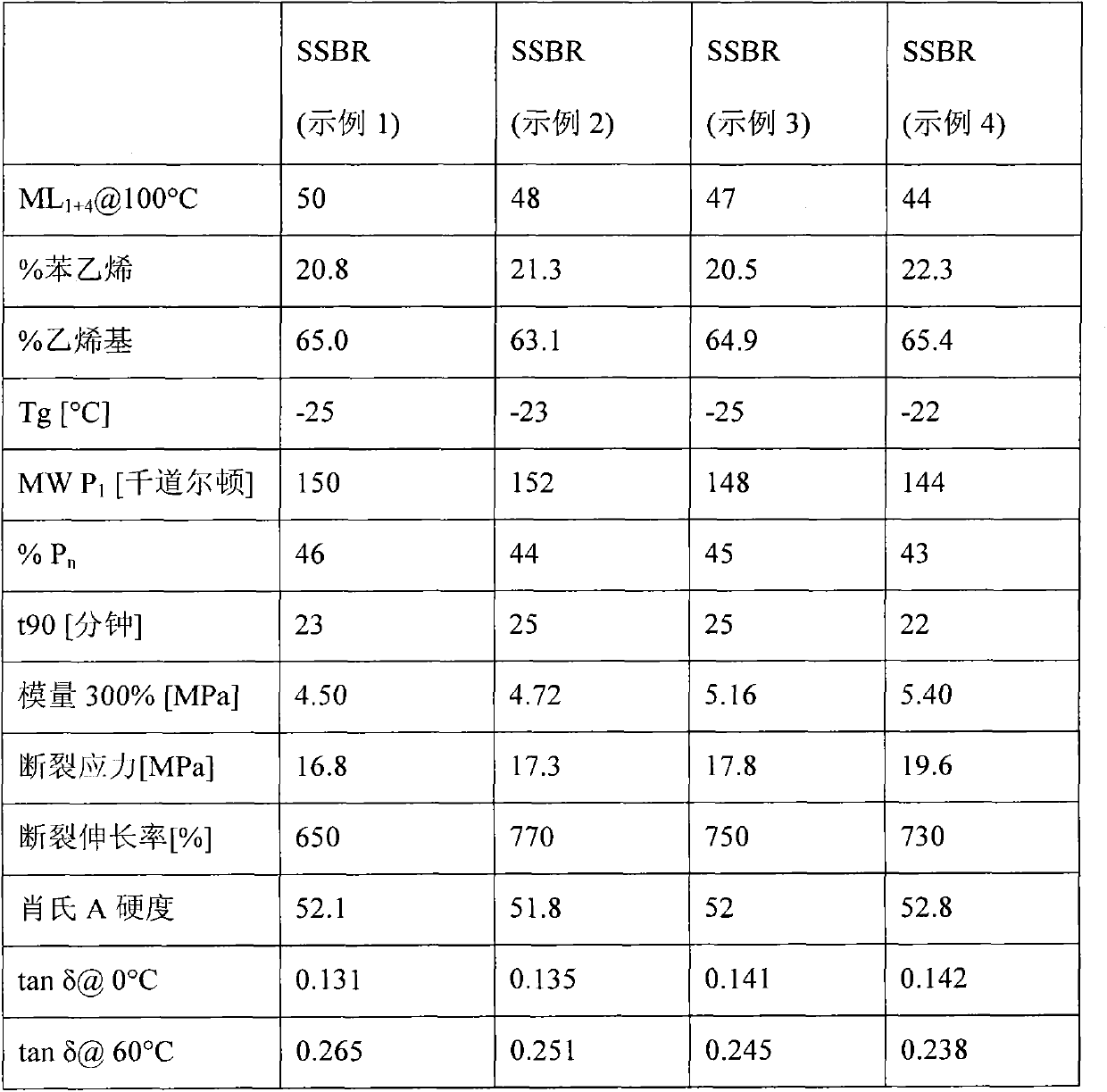
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
26 results about "Lithium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium (from Greek: λίθος, romanized: lithos, lit. 'stone') is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the lightest metal and the lightest solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride.
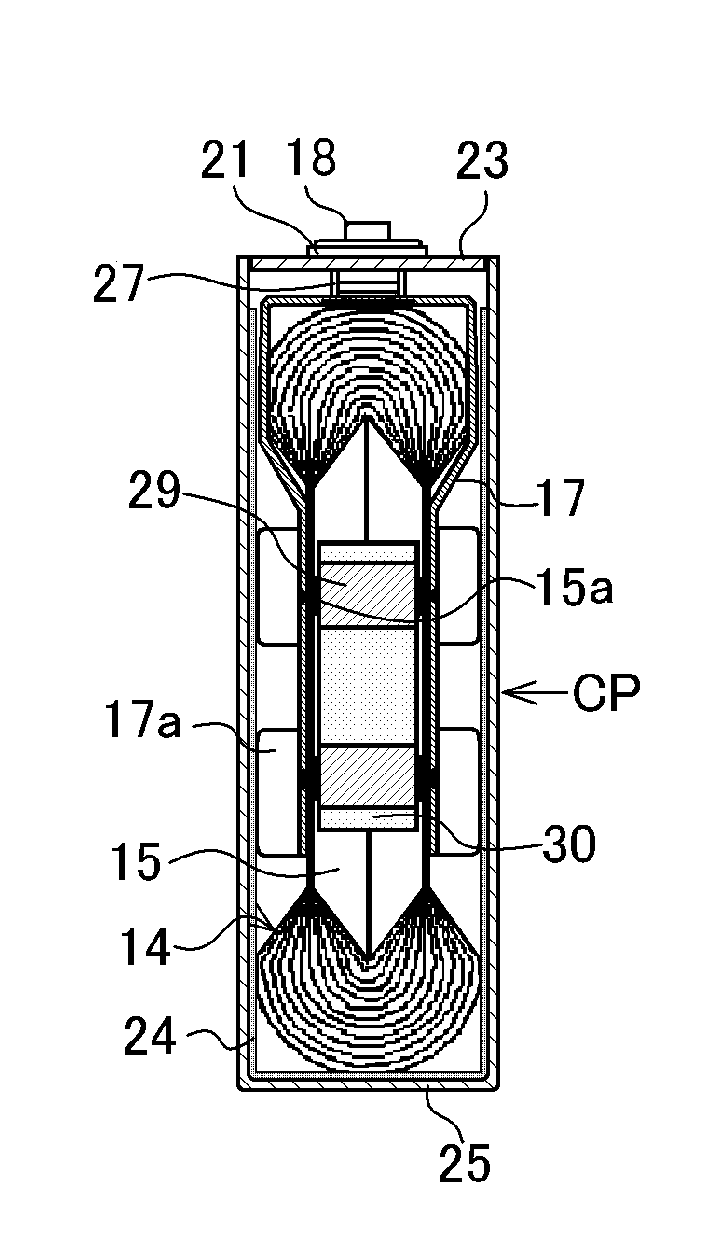
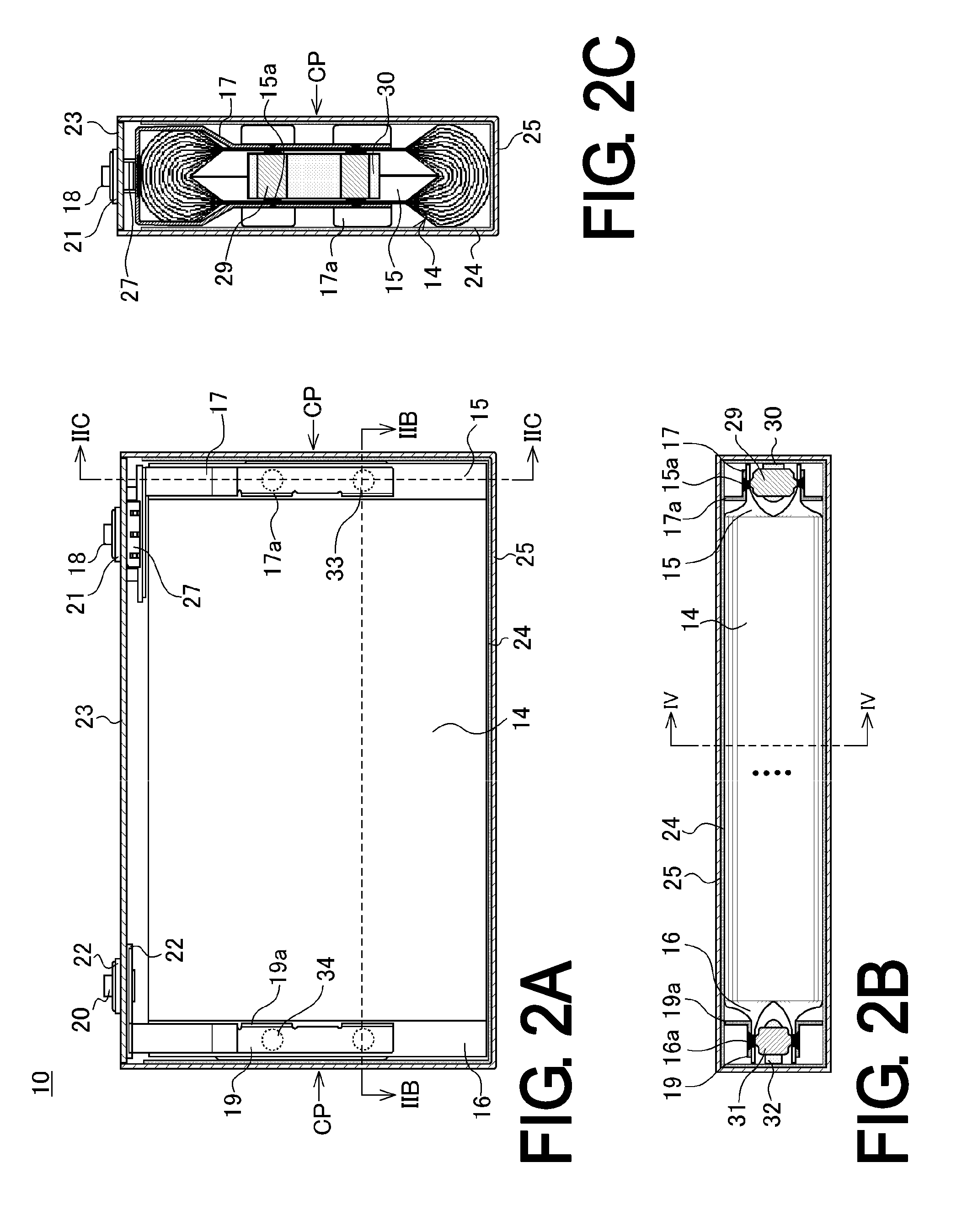
Nonaqueous-electrolyte battery
ActiveUS20090061303A1Improve securitySatisfactory battery characteristicCell electrodesCell component detailsSimple Organic CompoundsLithium
Owner:KK TOSHIBA
Coating modification method for improving performance of rich-lithium manganese-base positive electrode material
Owner:UNIV OF SCI & TECH BEIJING
Phosphor-contained solar cell and method thereof
InactiveUS20120204951A1Improve photoelectric conversion efficiencySemiconductor/solid-state device manufacturingPhotovoltaic energy generationChemistryLithium
Owner:WANG CHUNG YU +1
Low-cost high-strength dolomite earthen-ware green body and preparation method thereof
Owner:FUJIAN DEHUA FIVE CONTINENTS CERAMIC MFG CO LTD
Positive electrode for nonaqueous electrolyte secondary battery and nonaqueous electrolyte secondary battery
ActiveUS20170187036A1InhibitionWithout deteriorating battery characteristicFinal product manufactureElectrode carriers/collectorsLithiumInorganic particle
The positive electrode as an embodiment includes a positive electrode current collector mainly composed of aluminum, a positive electrode mixture layer containing a lithium-containing transition metal oxide and disposed above the positive electrode current collector, and a protective layer disposed between the positive electrode current collector and the positive electrode mixture layer. The protective layer contains inorganic particles, an electro-conductive material, and a binding material; is mainly composed of the inorganic particles; and is disposed on the positive electrode current collector to cover the positive electrode current collector in approximately the entire area where the positive electrode mixture layer is disposed and at least a part of the exposed portion of the positive electrode current collector where the positive electrode mixture layer is not disposed on the surface of the positive electrode current collector.
Owner:PANASONIC CORP
Na<+> superionic conductor (NASICON) type lithium-ion solid electrolyte collaboratively doping with F<->, B<3+> and Y<3+> ions and preparation method thereof
InactiveCN105140559ASmall hindranceReduce transmission bottleneckFinal product manufactureElectrolyte accumulators manufactureLithiumPhysical chemistry
Owner:XI'AN INST OF OPTICS & FINE MECHANICS - CHINESE ACAD OF SCI
Electrode assembly, manufacture method thereof and lithium secondary battery
InactiveCN103887472AEasy to processExtend your lifeFinal product manufactureLi-accumulatorsLithiumEngineering
The invention discloses an electrode assembly which comprises a first polar plate, and two second polar plates, wherein active material layers are coated on two surfaces of the first polar plate, the first polar plate is continuously bent into a Z shape of a vertical cross-section along the length direction of the first polar plate, an active material layer is coated on one surface of the second polar plate, each second polar plate is continuously bent into a Z shape of a vertical cross-section along the length direction of the second polar plate, two surfaces of the first polar plate, coated with the active material layers, are respectively opposite to surfaces of the two second polar plates, coated with the active material layers, an isolating layer is arranged between the surfaces of the first polar plate, which are opposite to the second polar plates, and the first polar plate and the second polar plates are respectively provided with a contact region with connected leads. The electrode assembly is low in requirement of production equipment, is easy to process and manufacture, and is relatively prolonged in service life. The material utilization rate of the electrode assembly can be increased, the cost is lowered, and the energy density of a battery is improved, so that the volume of the electrode assembly is relatively reduced under the same capacity.
Owner:INST OF IND TECH GUANGZHOU & CHINESE ACADEMY OF SCI +1
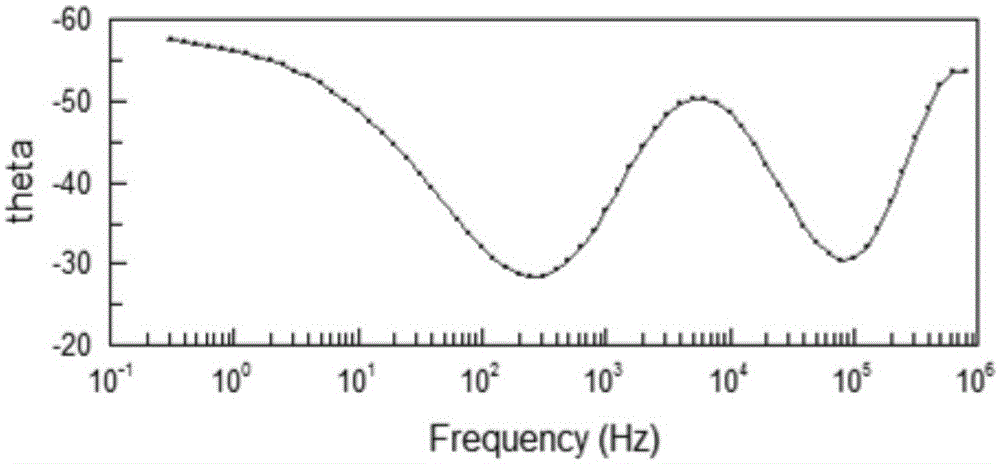
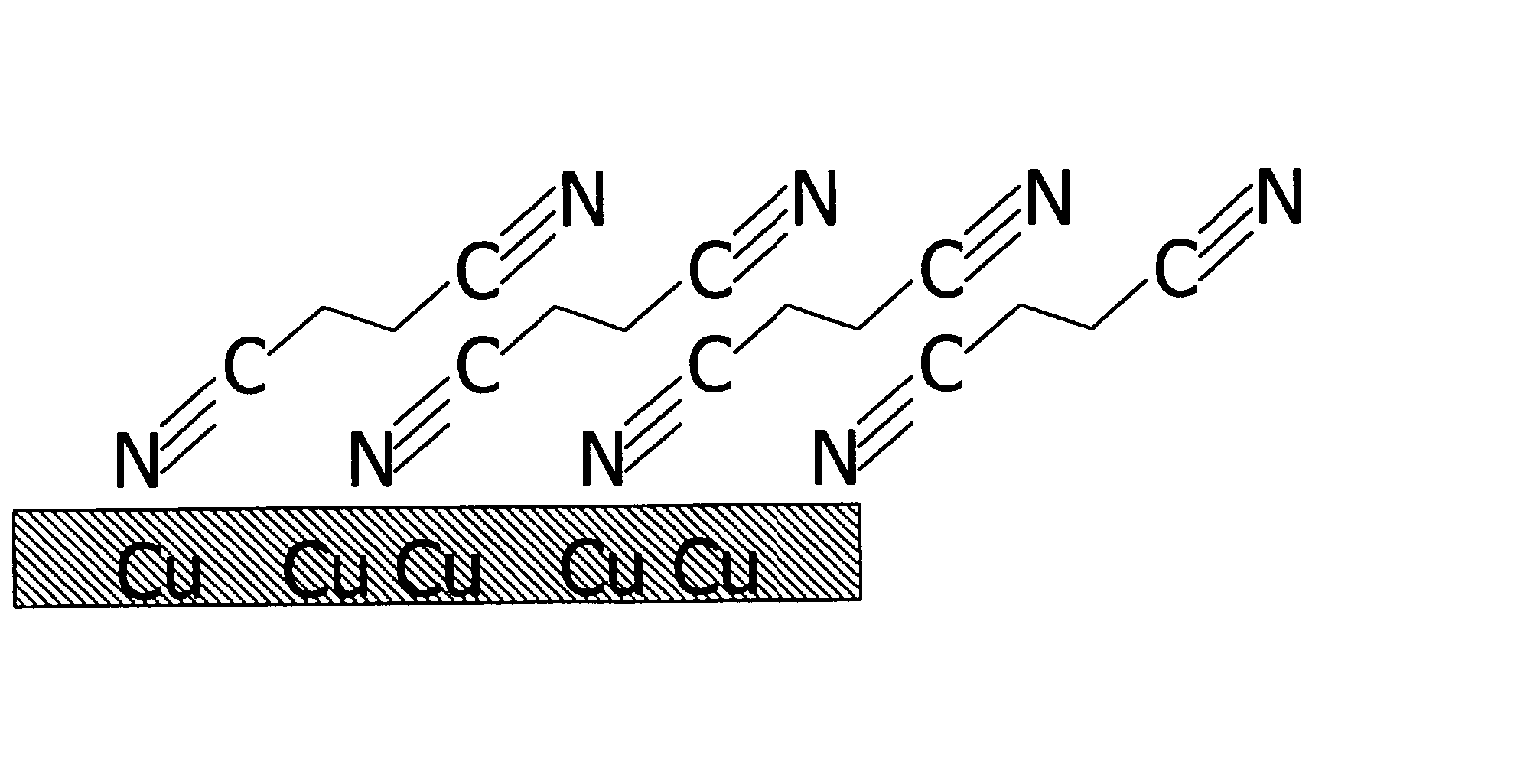
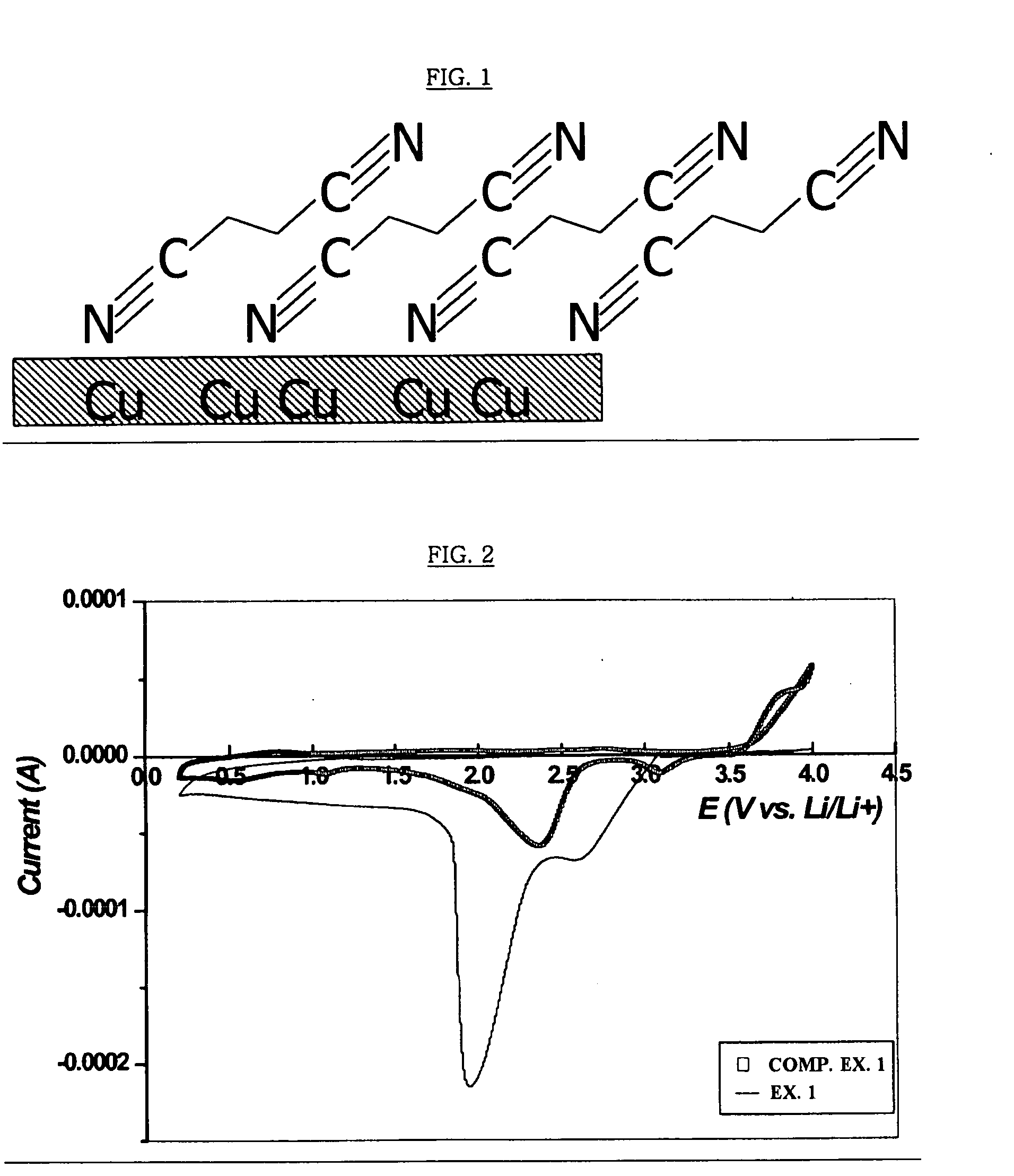
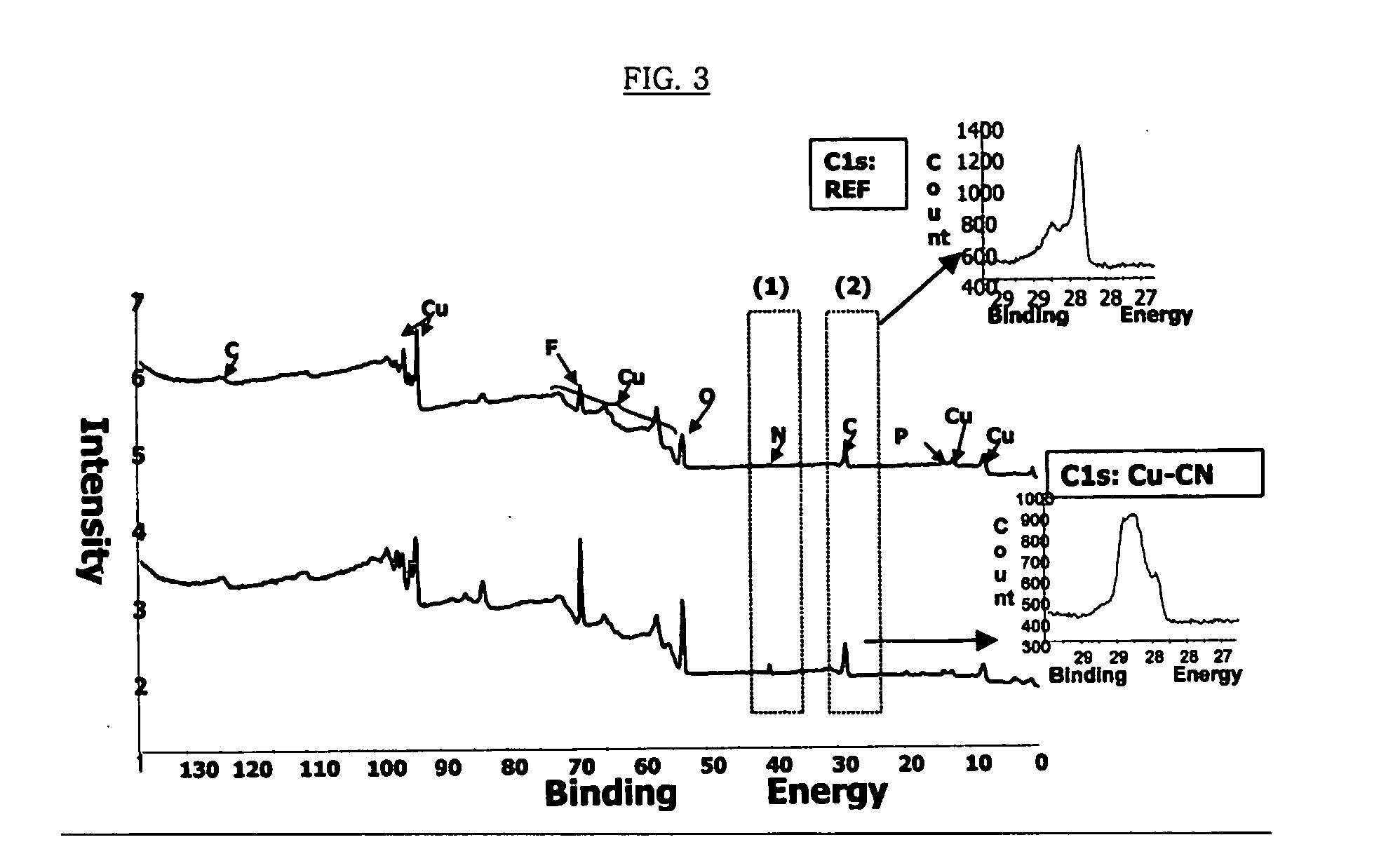
Copper collector for secondary battery comprising Cu-nitrile compound complex formed on surface thereof
ActiveUS20060147803A1Improve capacity restorabilityImprove battery qualityFinal product manufactureElectrode carriers/collectorsLithiumCopper foil
Owner:LG ENERGY SOLUTION LTD
Nonaqueous electrolyte secondary battery
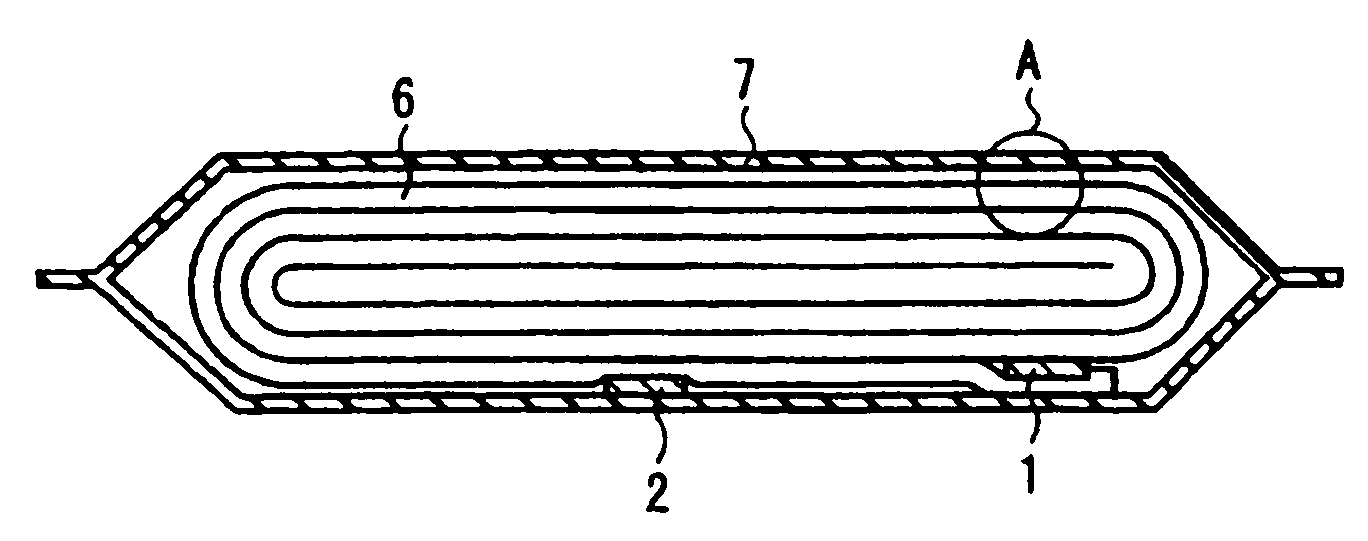
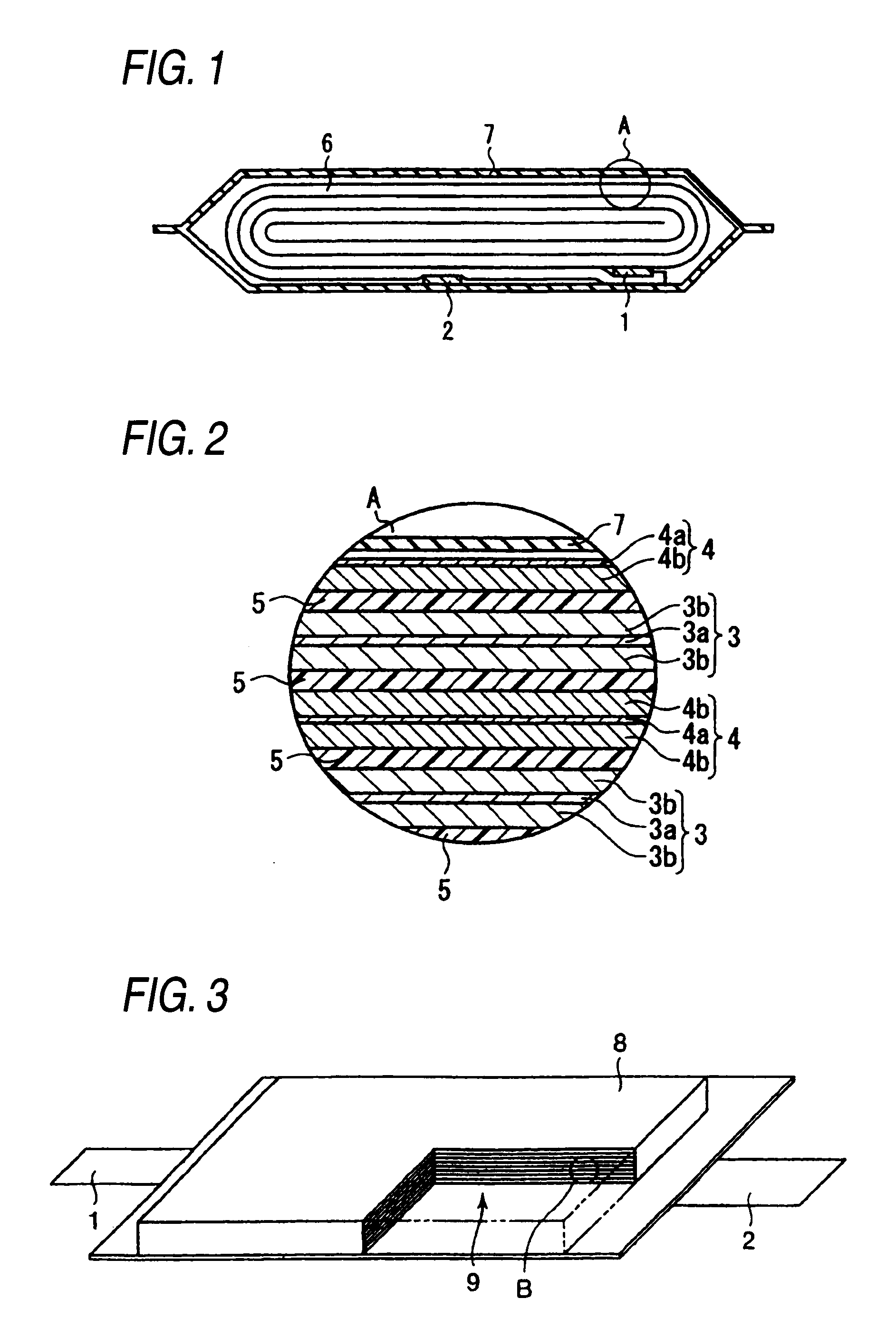
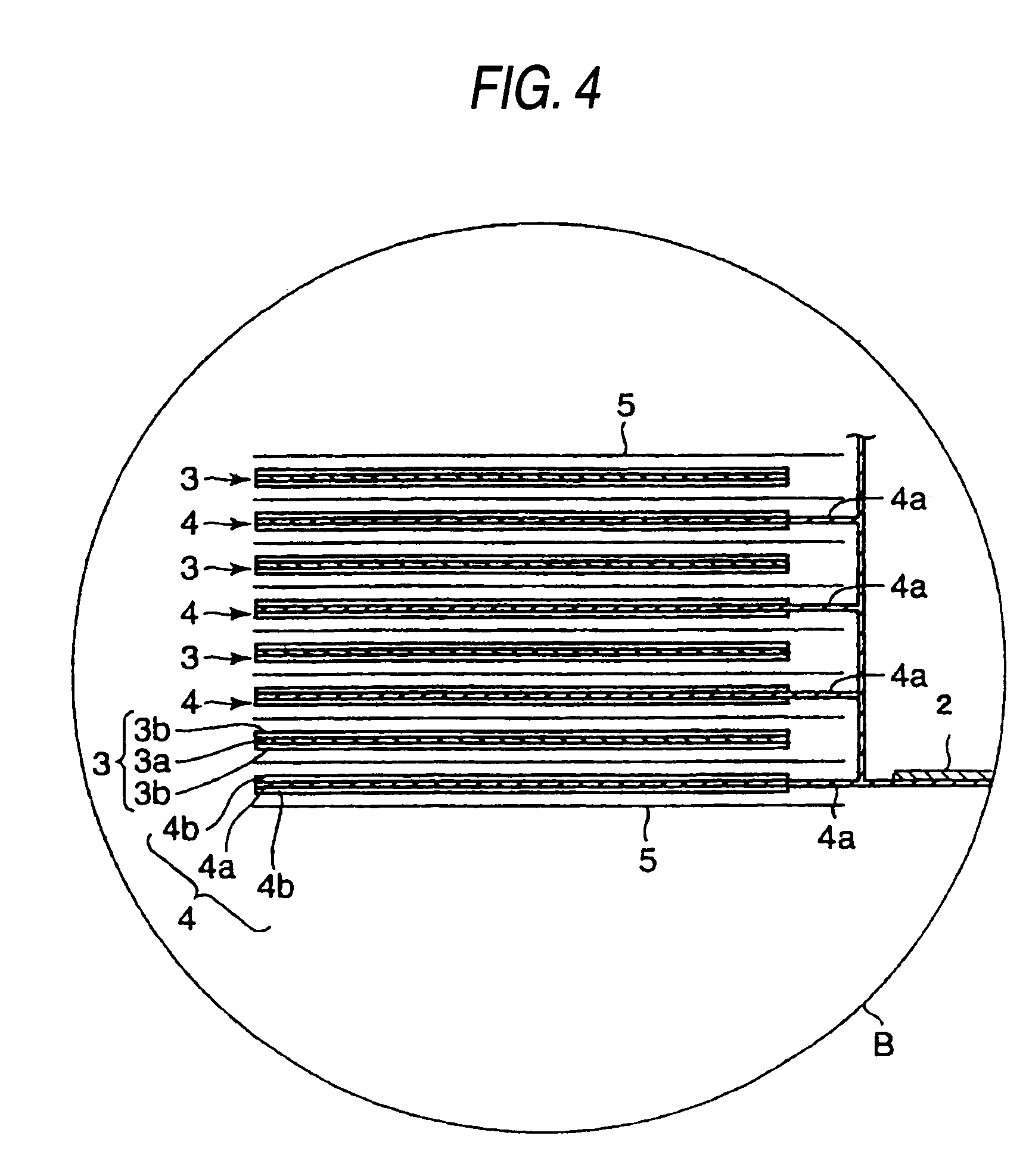
ActiveUS20140045015A1Better seePreferable characteristicFinal product manufactureWound/folded electrode electrodesElectrolyteLithium
A nonaqueous electrolyte secondary battery includes a flat winding electrode assembly including a positive electrode substrate exposed portion on one end and a negative electrode substrate exposed portion on the other end. The winding numbers of the positive and the negative electrode substrate exposed portions are each 30 or more. The positive and negative electrode substrate exposed portions each have an outermost surface welded and connected with a positive and a negative electrode collectors, respectively. A nonaqueous electrolyte used to fabricate the battery contains a lithium salt having an oxalate complex as an anion. At the welded connection portions, all of the layers of the positive electrode substrate exposed portion are melted to be welded and connected to the positive electrode collector, and all of the layers of the negative electrode substrate exposed portion are melted to be welded and connected to the negative electrode collector.
Owner:SANYO ELECTRIC CO LTD
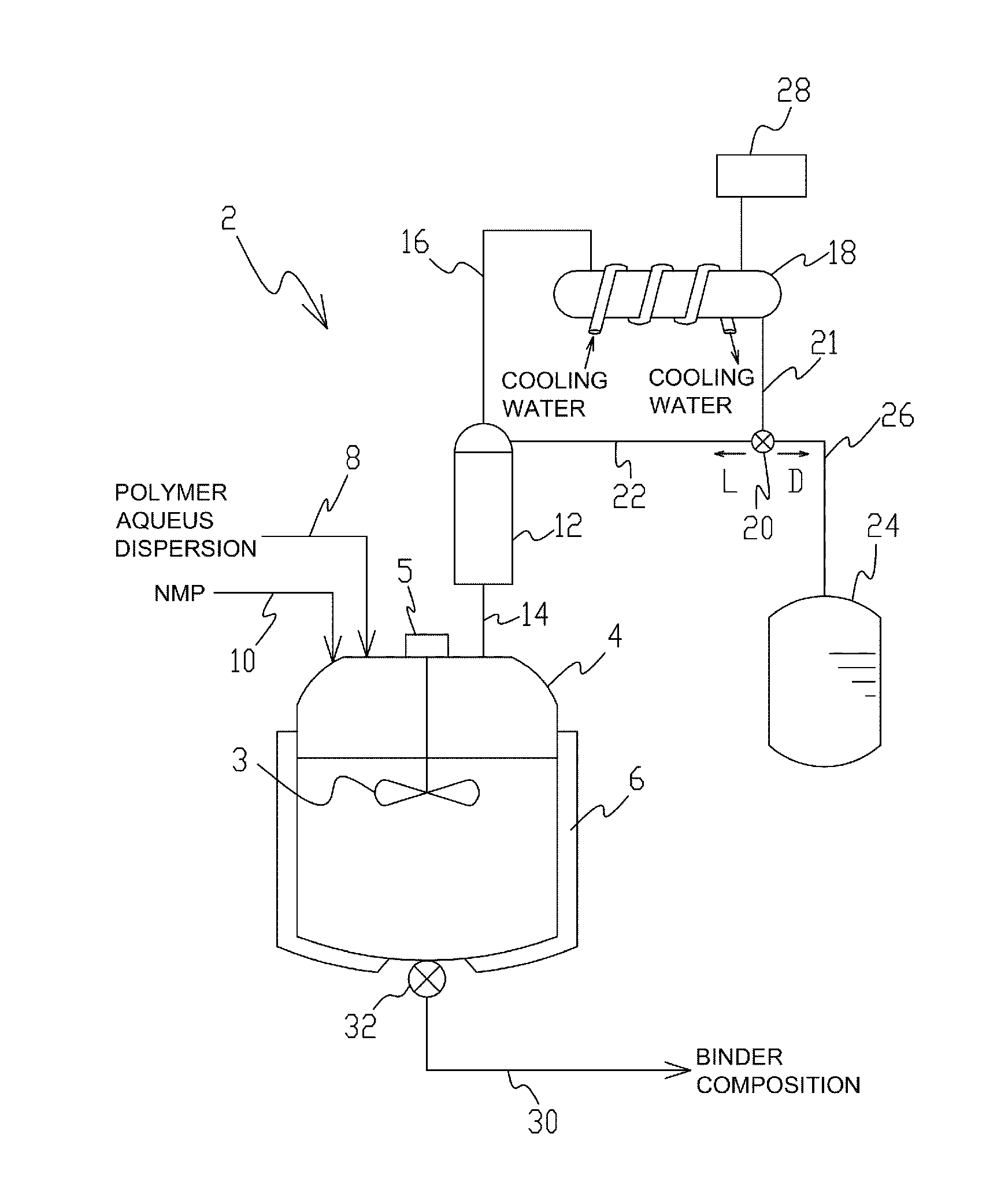
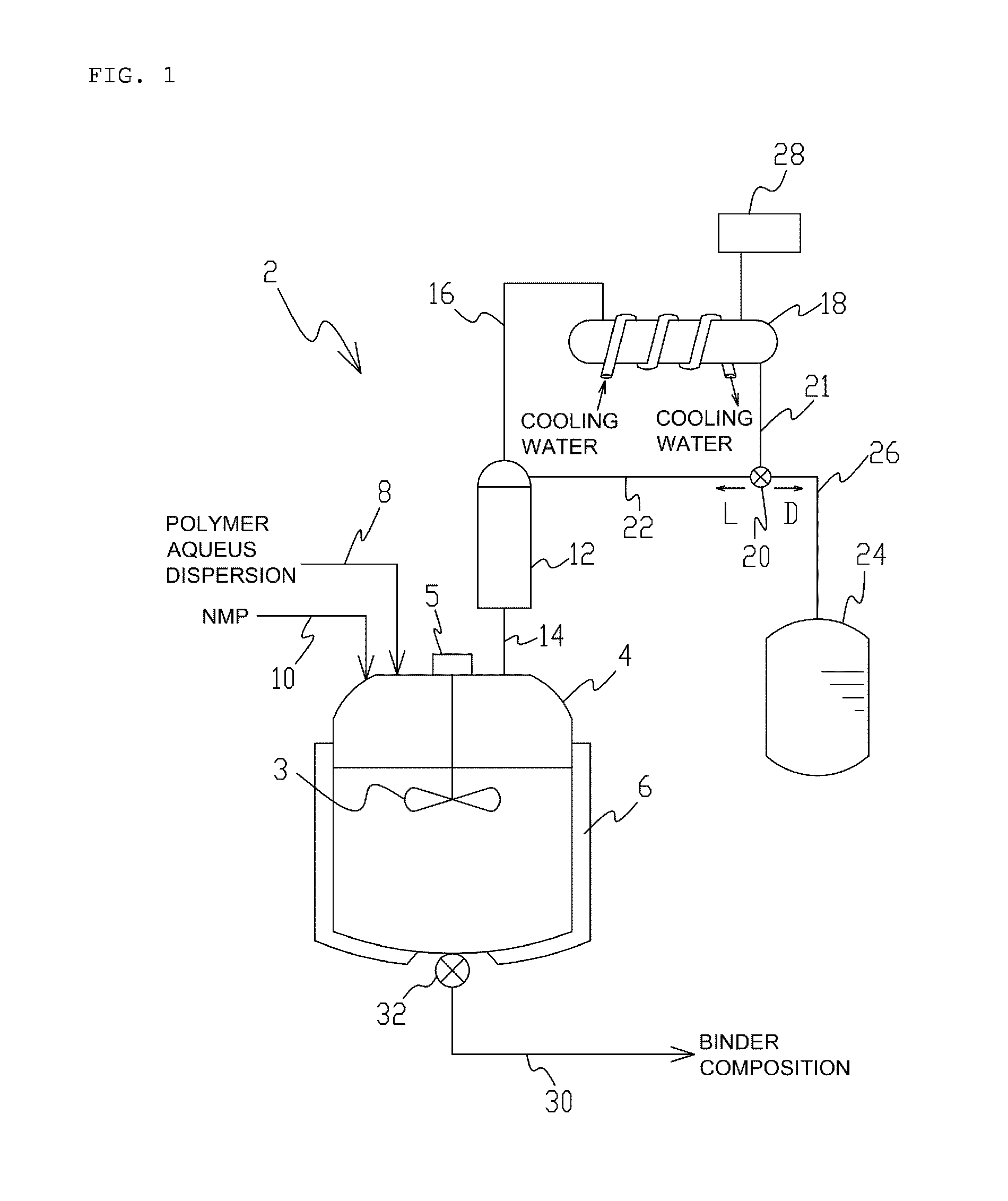
Method for producing slurry for heat-resistant layer for lithium ion secondary battery and method for producing electrode for lithium ion secondary battery
InactiveUS20140242296A1Reduce the amount of waterEfficiently obtainedElectrode manufacturing processesMixing methodsPolymer scienceDistillation
Owner:ZEON CORP
Lithium-rich anode material and preparation method thereof
Owner:MCNAIR TECH +1
Stainless steel crucible paint used for smelting aluminum-lithium alloy as well as preparation method thereof and coating method thereof
ActiveCN110172627AImprove stabilityImprove thermal shock resistanceMetallic material coating processesLithiumCrucible
Owner:NORTHEASTERN UNIV
Separator for lithium secondary battery, method for manufacturing same, and lithium secondary battery including same
ActiveUS20190189987A1Improve heat resistanceImprove performance and securityFinal product manufactureSecondary cellsPorous layerLithium
The present invention relates to a separator for a lithium secondary battery, a method for manufacturing the separator, and a lithium secondary battery including the separator. The separator includes a porous substrate and a heat resistance porous layer positioned on at least one surface of the porous substrate, wherein the heat resistance porous layer includes a sulfonic acid group-containing polysulfone.
Owner:SAMSUNG SDI CO LTD +2
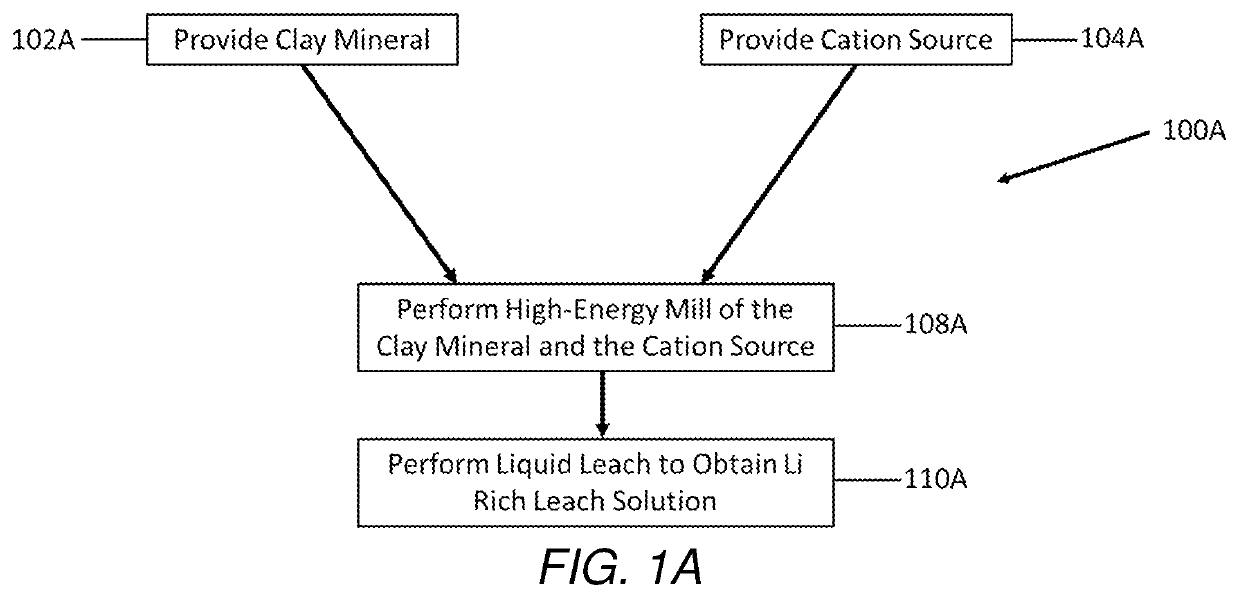
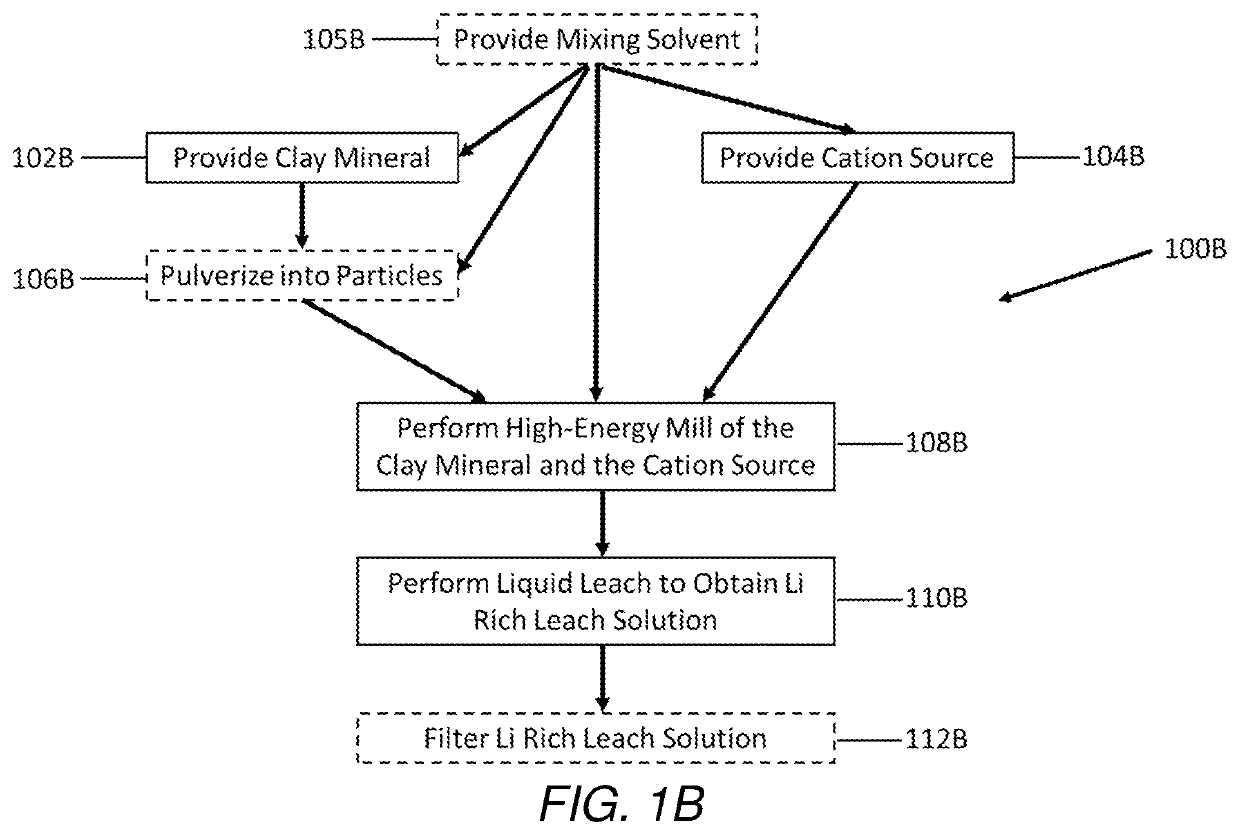
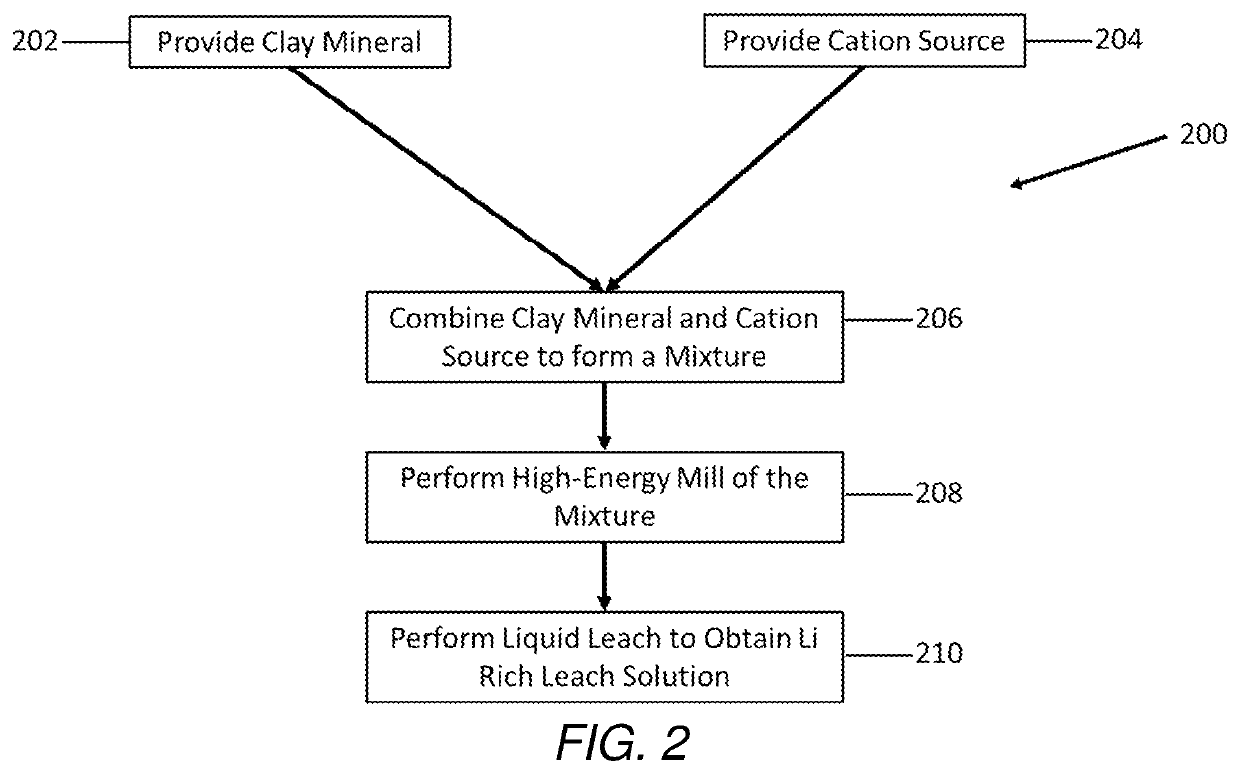
Selective extraction of lithium from clay minerals
Processes for extracting lithium from a clay mineral and compositions thereof are described. The extraction process includes providing a clay mineral comprising lithium, mixing a cation source with the clay mineral, performing a high-energy mill of the clay mineral, and performing a liquid leach to obtain a lithium rich leach solution.
Owner:TESLA INC
Comb polymer electrolyte and preparation and application thereof
ActiveCN107658501AImprove mechanical propertiesHigh thermodynamic stabilityFinal product manufactureElectrolyte accumulators manufactureLithiumPolymer electrolytes
Owner:HUAZHONG UNIV OF SCI & TECH
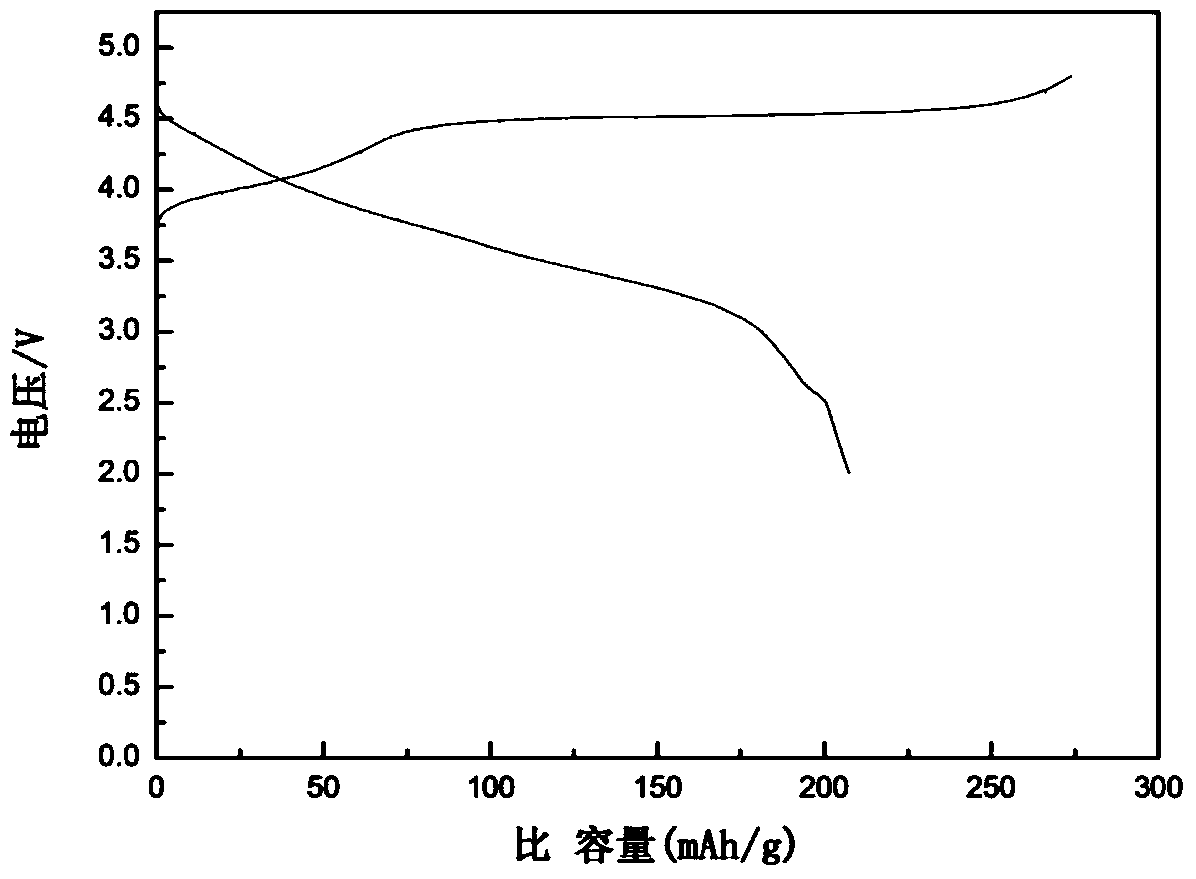
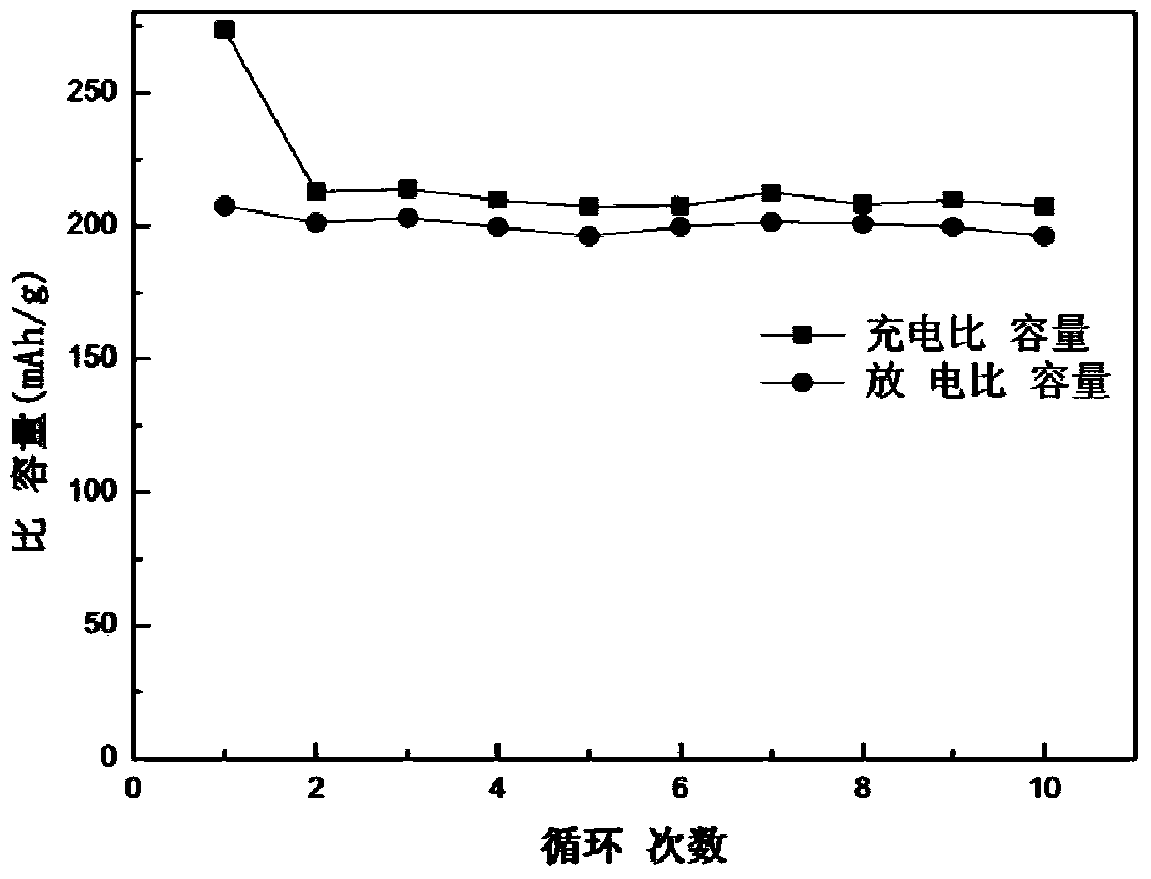
Method of preparing positive electrode active material for lithium secondary battery and positive electrode active material for lithium secondary battery prepared thereby
ActiveUS20190123350A1Increase capacityImproving rate propertySecondary cellsPositive electrodesOxideLithium
Owner:ECOPRO BM CO LTD
Special magnesium alloy material for vehicle crankshaft and preparation method thereof
The invention discloses a special magnesium alloy material for a vehicle crankshaft and a preparation method thereof. The special magnesium alloy material for the vehicle crankshaft comprises the following components in percentage by weight: 4.0-7.0% of silicon, 3.7-5.8% of copper, 2.5-4.0% of lithium, 0.4-0.6% of tungsten, 0.5-0.9% of chromium, 0.04-0.07% of titanium, 0.02-0.07% of boron, 0.001-0.004% of nickel, 0.001-0.003% of vitriol, 0.001-0.003% of zirconium, and the balance of magnesium. The high-performance magnesium alloy material with higher strength and hardness is prepared through selecting the specific formula, has the advantages of lightness and wear resistance, is excellent in heat dissipating capacity, and can satisfy the quality requirement of the vehicle crankshaft.
Owner:YUYAO WANZHEN HARDWARE FACTORY
Corrosion-resistant alloy
InactiveCN103265751ACorrosion resistant alloyLithium
The invention relates to a corrosion-resistant alloy, consisting of main components and auxiliary components, wherein the main components consist of the following components in percentage by mass: 32-38% of magnesium, 2-6% of molybdenum, 15-18% of lithium, 2-5% of cobalt, 18-23% of nickel and 2-5% of vanadium; and the auxiliary components consist of the following components in percentage by mass: 15-25% of ethylene-tetrafluoroethylene copolymer, and 1-2% of a lubricant. The corrosion-resistant alloy has good corrosion resistance.
Owner:CHUZHOU HAOYU SLIDING BEARING CO LTD
Process for the preparation of 1,3-butadiene and styrene copolymers and use thereof in vulcanizable elastomeric compositions
Owner:VERSALIS SPA
Purified quartz powder modified for cladding optic fiber cable
A highly purified quartz powder having a low level of naturally occurring lithium modified for cladding a fiber optic cable, said modified quartz powder having an increased total amount of lithium in solid solution in said powder, said increased total amount being in the range of more than 0.50 ppm and less than 1.00 ppm and a method of modifying an highly purified quartz powder to make the same.
Owner:SIBELCO NORTH AMERICA INC
Zr-containing flame-retardant aluminum-lithium alloy and processing technology thereof
Owner:GUANGZHOU YUZHI TECH CO LTD
Composition for preparing electrode material
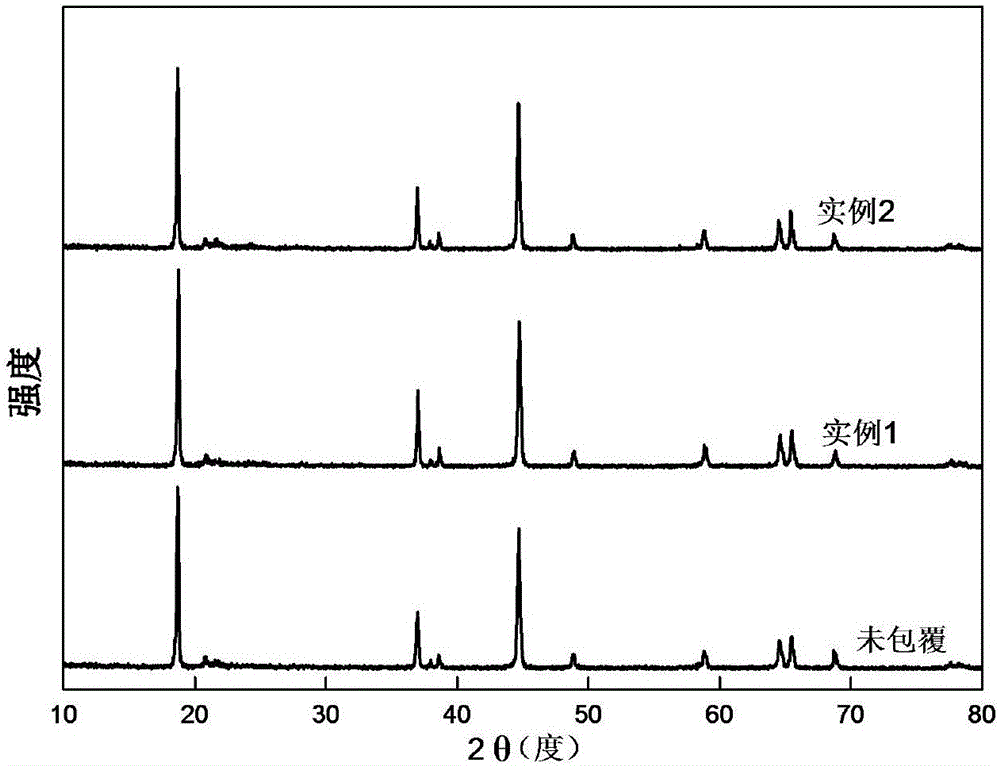
A nickel-based hydroxide powder having an average crystallite size of at most 10 nm, as determined by Scherrer fitting of the (00I) reflections of the XRD powder diffraction pattern of the nickel-based hydroxide powder, and a method for producing the nickel-based hydroxide powder are provided. The nickel-based hydroxide powder can be used as a precursor for forming a lithium transition metal oxide active electrode material.
Owner:EV METALS UK LTD
Preparation method of multi-phase catalyst for synthesizing dimethoxy bisphenol bimethyl carbonate
ActiveCN108816213AImprove conversion rateHigh selectivityPreparation from organic carbonatesMetal/metal-oxides/metal-hydroxide catalystsMethyl carbonateHigh pressure
The invention discloses a preparation method of a multi-phase catalyst for synthesizing dimethoxy bisphenol bimethyl carbonate. The preparation method comprises the following steps: (1) dissolving polyvinyl pyrrolidone into deionized water, and stirring for fully dissolving the polyvinyl pyrrolidone, thus obtaining a solution A; (2) adding a lithium source and a titanium source in the solution A one by one or at the same time, ultrasonically stirring for 30 to 60 minutes, and then stirring for 6 to 12 hours, thus obtaining a solution B, wherein a molar ratio of the lithium source to the titanium source is 1:1 to 1:10; (3) transferring the solution B into a high-pressure reaction kettle, putting the high-pressure reaction kettle at 150 to 180 DEG C, and reacting for 3 to 6 minutes with pressure; opening the high-temperature reaction kettle after cooling, evaporating a solvent, and drying obtained solid at 80 to 120 DEG C for 6 to 12 hours, thus obtaining white solid; (4) roasting the white solid in a muffle furnace of which the temperature is 300 to 600 DEG C for 2 to 6 hours, thus obtaining the multi-phase catalyst. A Ti / Li bimetal catalyst is prepared for the first time, and the Ti / Li bimetal catalyst is used in transesterification reaction of bisphenol A and dimethyl carbonate for the first time, so that the conversion rate of the bisphenol A is increased, the selectivity oftransesterification is increased, and alkylated products are reduced.
Owner:TIANJIN POLYTECHNIC UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap