Process for the preparation of 1,3-butadiene and styrene copolymers and use thereof in vulcanizable elastomeric compositions
A technology of styrene and copolymer, applied in the field of preparation 1, can solve problems such as poor compatibility and unsatisfactory performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 (comparison)
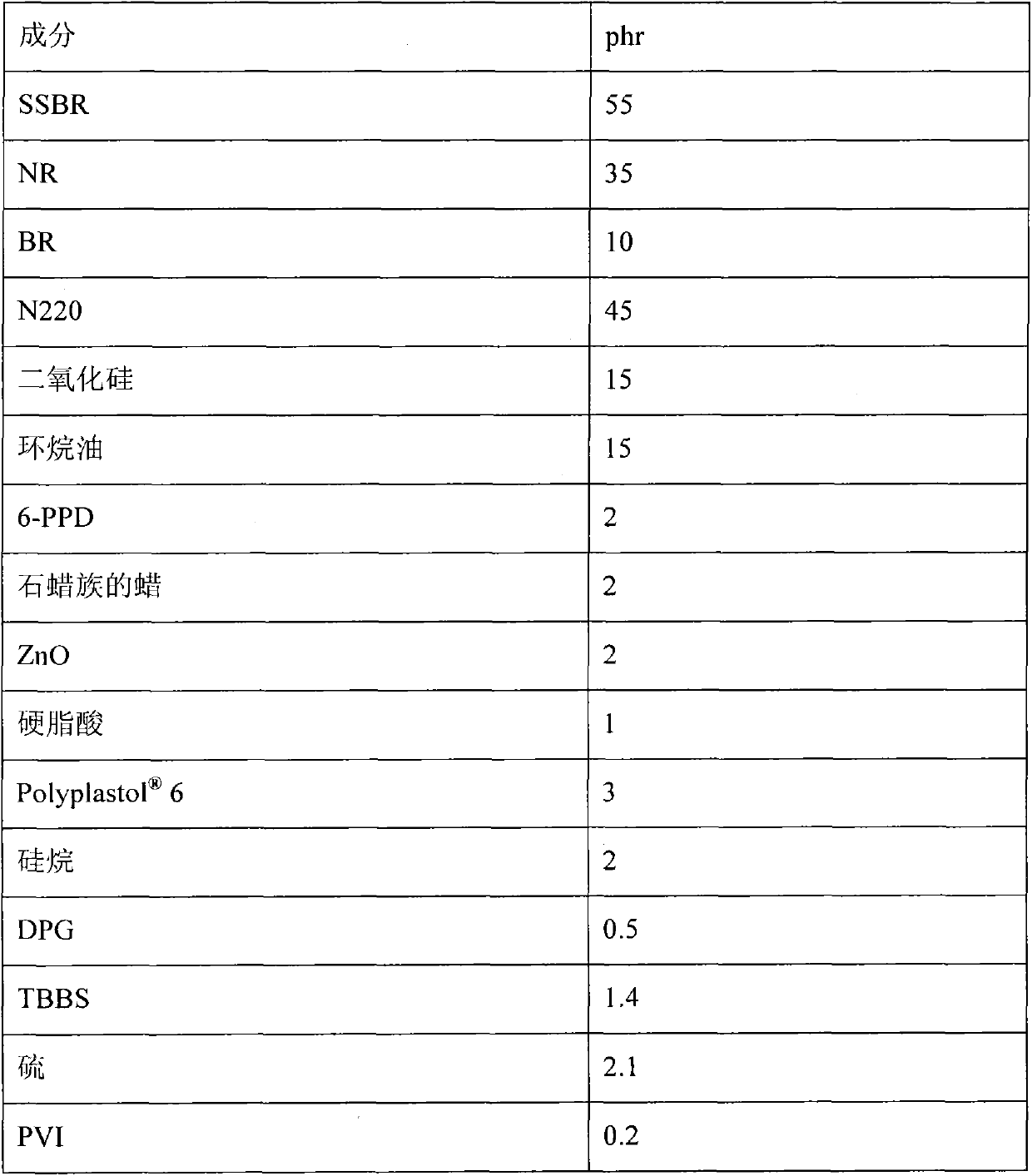
[0048] A dry mixture of 8000 g of cyclohexane / hexane (9 / 1 by weight, equal to a filling factor of 80%), 3.12 g of a polar modifier (tetrahydrofurfuryl ethyl ether-THFAethyl) (corresponding to 255 ppm , to the theoretical amount of initiator in a molar ratio of about 3:1) and then 300 g of styrene and 840 g of 1,3-butadiene were fed into a 16-liter stirred reactor. The resulting reaction mixture was heated to a temperature of 40°C by means of a heating jacket. Then, 0.51 g of n-butyllithium in n-hexane (3.41 g of a 15% by weight solution) were fed. The heating of the jacket was then removed, and due to the exothermic nature of the reaction, an increase in the temperature of the reaction mixture occurred until a final temperature of about 76°C (peak temperature). After waiting 10 minutes after reaching the peak temperature, 60 g of 1,3-butadiene (capped) were fed so that all active ends were of the butadienyl type. After waiting an additio
Embodiment 2
[0049] Embodiment 2 (comparison)
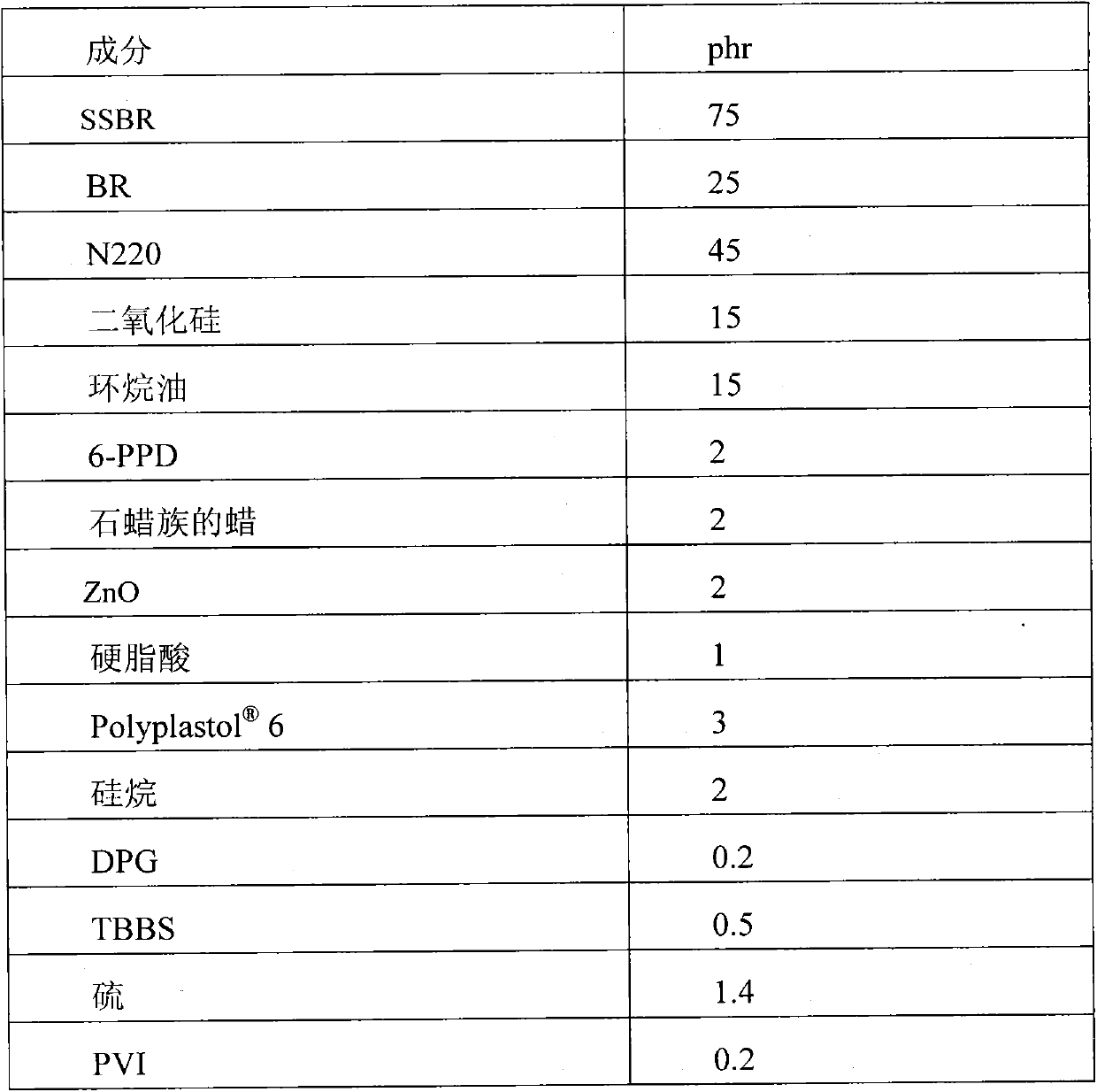
[0050] In a stirred 16 liter reactor, the same procedure as for the feeding of the reagents described in Example 1 was carried out up to the introduction of the coupling agent, which in the case of this example was tin tetrachloride in an amount equal to 0.261 g , corresponding to a theoretical coupling efficiency of 50%. The remaining stages of the reaction are the same as those described in Example 1.
Embodiment 3
[0051] Embodiment 3 (comparison)
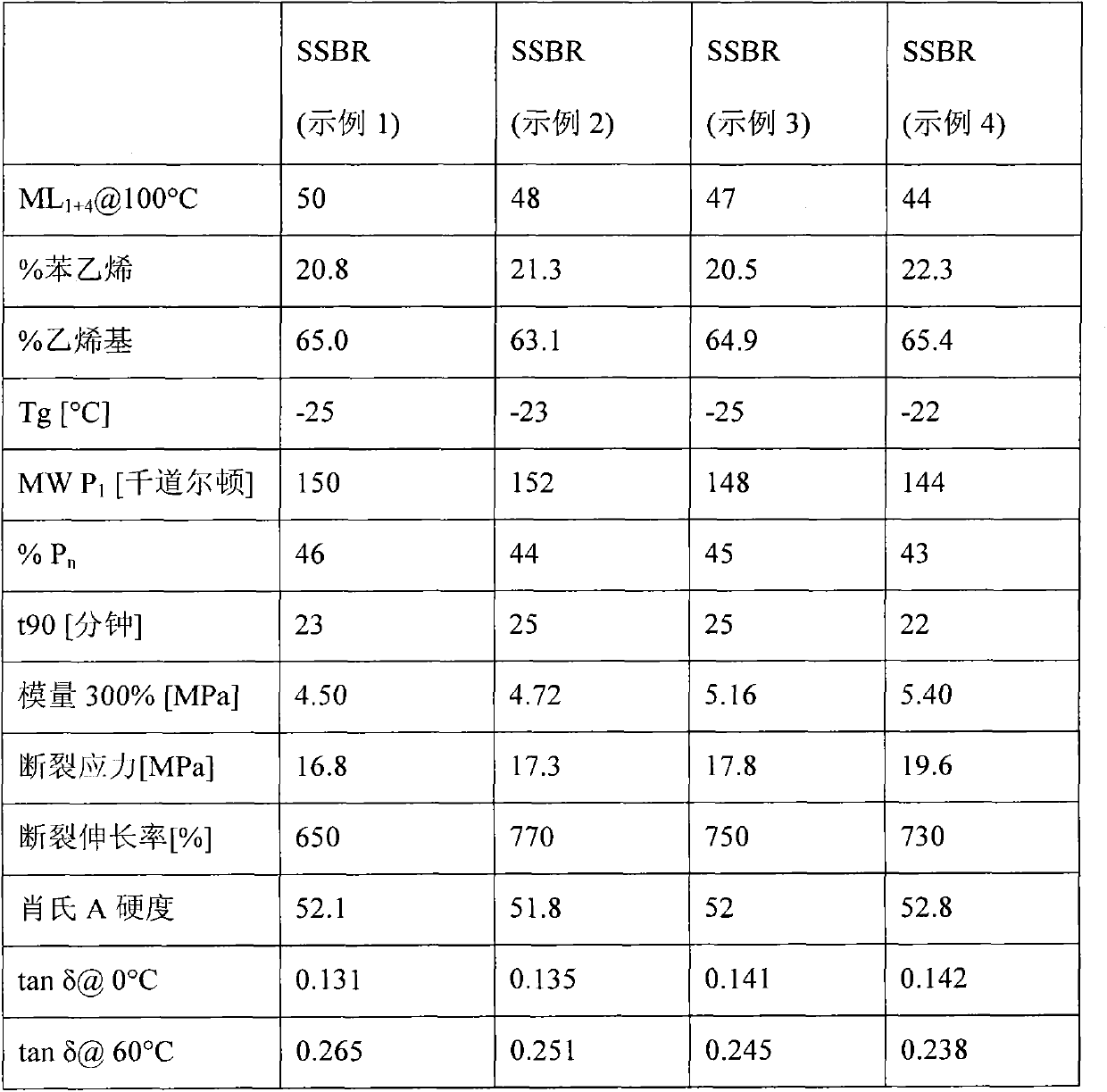
[0052] In a stirred 16 liter reactor, the same procedure as for the feeding of the reagents described in Example 1 was carried out until the coupling reaction with 0.261 g of tin tetrachloride was completed. After 5 minutes, 1.30 g of trioctyltin chloride was fed to deactivate the remaining active ends. After 10 minutes, the polymer solution was then drained into a tank where it was stabilized with 0.7 phr of 2,6-di-tert-butylphenol (BHT) and the entire solution was stripped with water to the desolvation section .
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap