Cefmetazole crystal-form compound and preparation method thereof
A technology of cefmetazole crystal and cefmetazole, which is applied in the field of cefmetazole crystal compound and its preparation, can solve the problems of cefmetazole physical and chemical properties, affecting the long-term stability of the drug, and affecting the safety of clinical medication, etc. Achieve the effect of high quality stability, high safety and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation method of cefmetazole crystal form compound of the present invention
[0046] Put 20g of crude cefmetazole into a three-necked flask at 6°C, add 80mL of acetone and 5mL of water and stir to dissolve, add 2 parts by mass of imported activated carbon and stir for 30 minutes, filter, adjust the pH of the solution to 1.5-2.5 with 30% phosphoric acid, and control The temperature of the solution is 25°C. Under the stirring speed of 150 revolutions per minute, slowly add 70 mL of 2-butanone dropwise at a speed of 8 mL / min until the crystals are precipitated, and then under the stirring speed of 80 revolutions per minute, control the process of the solution. Saturation, continue to add 50mL purified water, make crystallization completely, filter, wash twice with 20mL ethyl acetate, suck dry, dry under reduced pressure, obtain cefmetazole acid crystalline form compound 16.6g, yield is 83.0%, (purity 99.6%, melting 149.8℃)
[0047] The following are
Embodiment 11
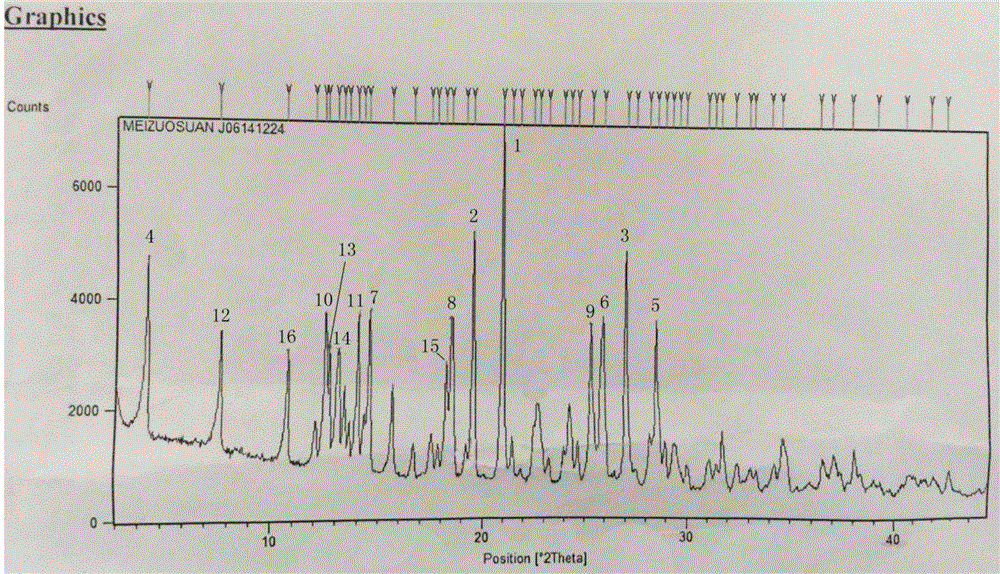
[0051] Example 11 The powder diffraction (PXRD) pattern of cefmetazole crystal form compound of the present invention
[0052] The crystal parameters of the cefmetazole crystal form compound prepared in Example 1 were determined by X-ray powder diffraction, and the X-ray powder diffraction conditions of the cefmetazole crystal form compound of the present invention were: D / max-3A type X-ray diffraction Instrument, measured 2θ under the conditions of Cu target Kα1 ray, tube current voltage: 40kv, current: 40mA, 25°C / min, range: 3°-45°.
[0053] Table 1 Example 1 of the present invention cefmetazole powder diffraction (PXRD) spectrum peak intensity
[0054]
[0055]
[0056] figure 1 It is the powder diffraction (PXRD) spectrum of the cefmetazole crystal form compound of the present invention. The numbers 1-16 are diffraction peaks, and Table 2 shows the 2θ and peak intensity of the cefmetazole crystal form compound of the present invention. Comprehensively obtained in
Embodiment 12
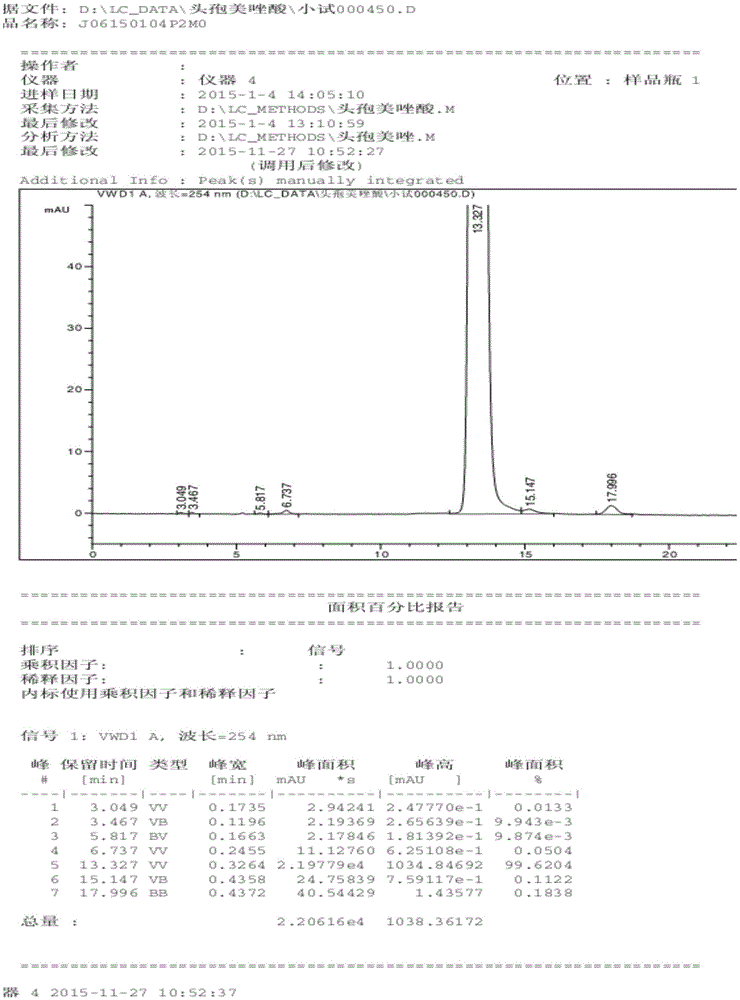
[0057] Example 12 Quality comparison of cefmetazole crystal form compound of the present invention and commercially available cefmetazole standard substance
[0058] The cefmetazole prepared by Examples 1, 2, and 3 of the present invention correspond to batches 1, 2, and 3 respectively, and the test results of key quality indicators are shown in Table 3.
[0059] Table 3 The quality comparison of cefmetazole crystal form compound of the present invention and commercially available cefmetazole standard substance
[0060]
[0061]
[0062] From the data analysis of Table 1, it can be seen that the prepared cefmetazole part key indicators of the present invention are more excellent in quality compared with the current commercially available standard substance (purchased from China Inspection Institute, product batch number 130580-201301), and its dissolution rate Faster, the content of related substances is obviously reduced, and its melting point is slightly higher than the s
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap