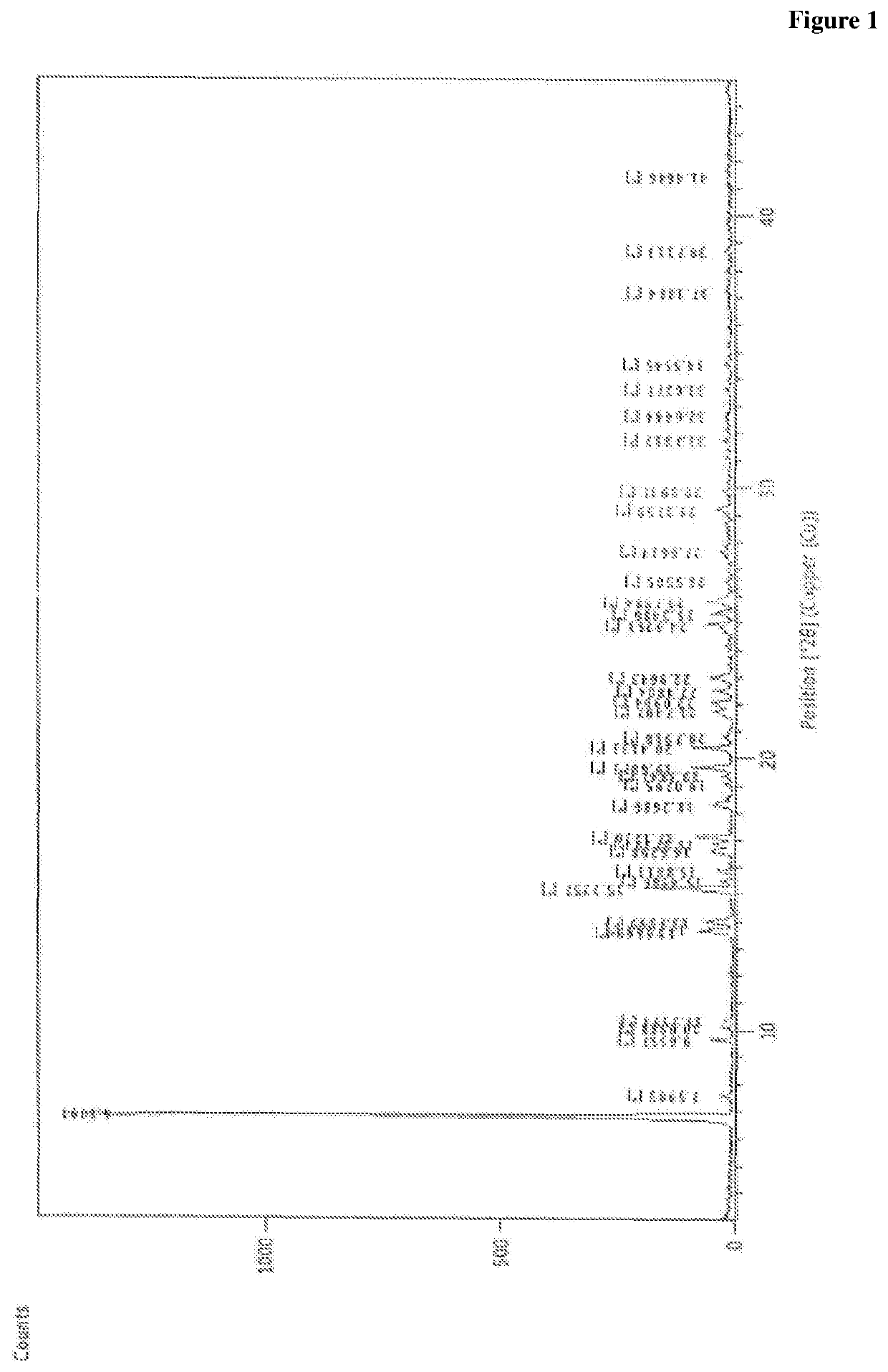
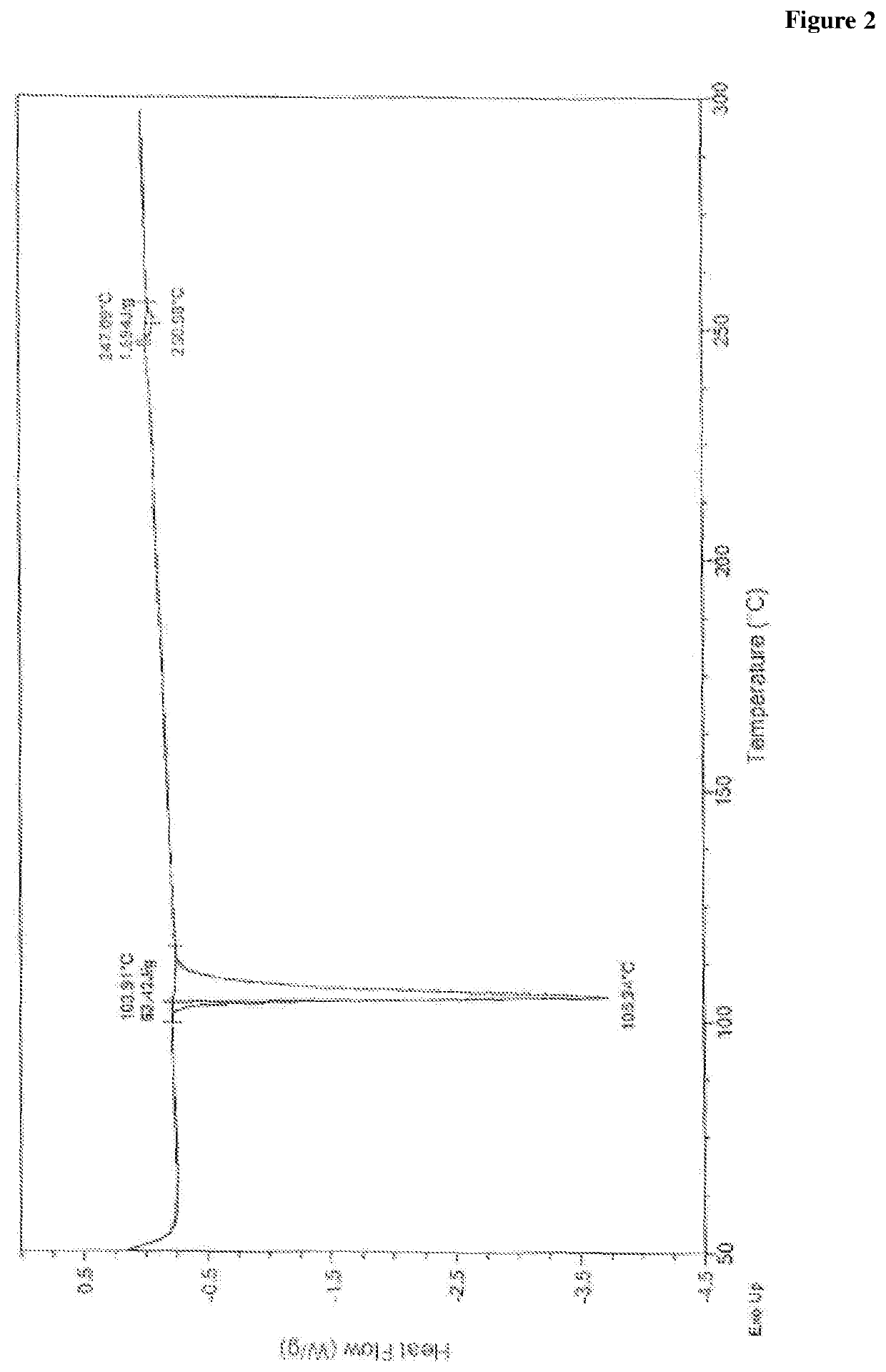
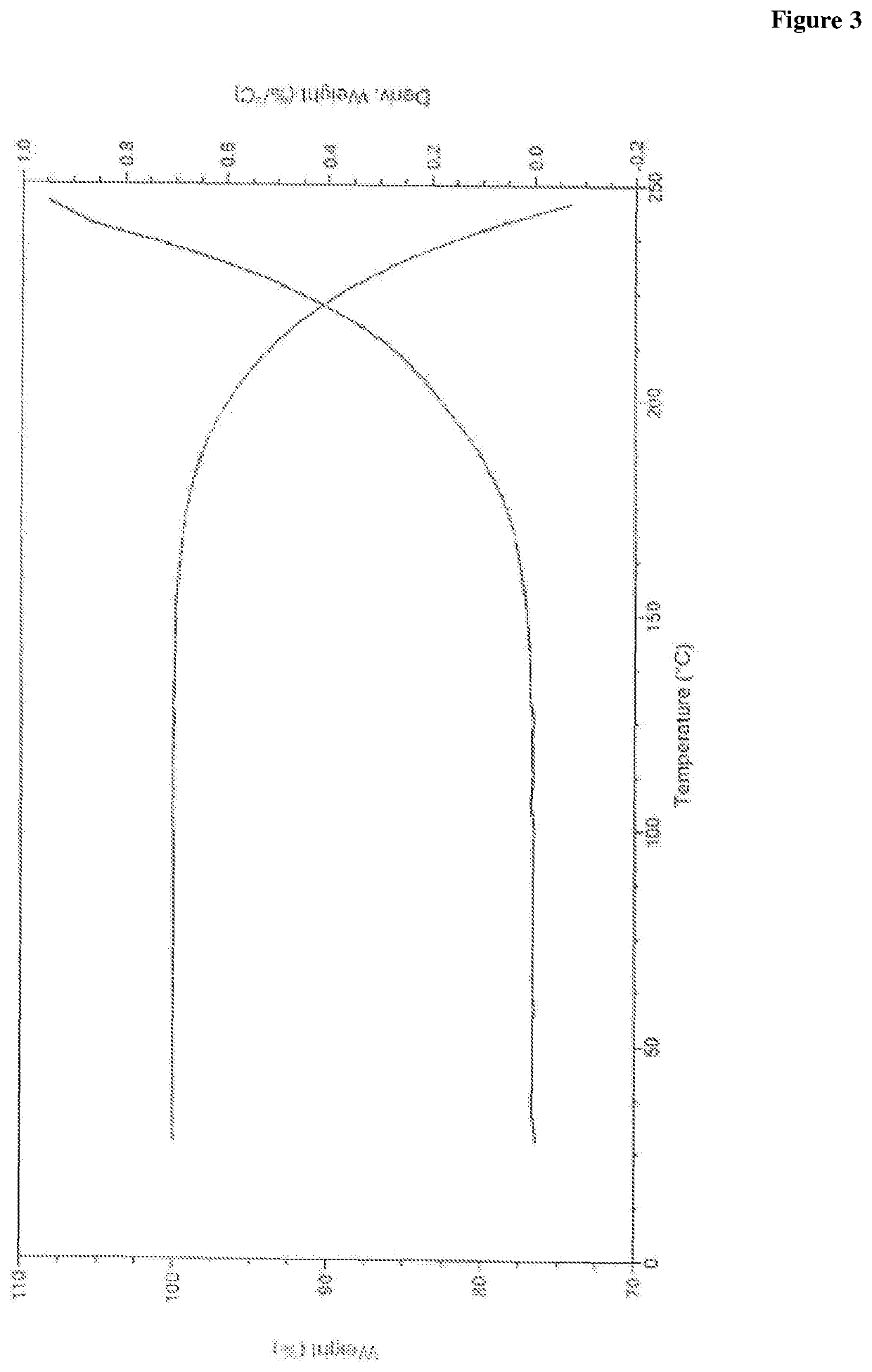
Novel process for preparation of empagliflozin or its co-crystals, solvates and their polymorphs thereof
a technology of empagliflozin and cocrystals, applied in the field of process for the preparation of empagliflozin or its cocrystals, solvates and/or polymorphs thereof, can solve the problems of high cost, difficult large-scale implementation, and insatiable purity of the empagliflozin obtained from the above process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 5
on of Compound of Formula III
[0289]The residue of compound of Formula IV was dissolved in a mixture of acetonitrile (300 ml) and dichloromethane (300 ml) at 25-35° C. Triethylsilane (129.2 gr) was added at 25-35° C. and the temperature of the reaction mass was cooled to −25 to −35° C. Borontrifluoride-diethyletherate (158.1 gr) was added to the reaction mass at −40 to −30° C. and stirred for 15 to 20 min. Temperature of the reaction mass was raised to 0° C. and further maintained with stirring for 3 to 4 hrs. Water was added, and the layers were separated and the aqueous layer was extracted with 2-methyltetrahydrofuran or ethyl acetate, combined the organic layers and washed with water, 8% sodium bicarbonate solution and further with 10% brine solution and dried over sodium sulphate. The organic layer was distilled completely under vacuum at below 45° C. and co-distilled with n-heptane and purified by ethyl acetate to obtain the title compound as a solid.
Yield: 40 gms
example 6
on of Empagliflozin of Formula I
[0290]The residue of compound of Formula III was dissolved in N,N-Dimethylformamide (210 ml) at 25-35° C. Cesium carbonate (74.5 gr) and R-Tosyl tetrahydrofuran (26.8 gr) was added. Raised the temperature of the reaction mass to 45° C. to 50° C. and maintained for 12-24 hrs. Water and dichloromethane (525 ml) were added to the reaction mass and adjust the pH of the reaction mass to 7.0 to 8.0 using 5% hydrochloric acid solution. Separate the layers and extract the aqueous layer with dichloromethane. Organic layers were combined and washed with water, 10% sodium chloride, and dried over sodium sulphate. Filtered the solid and dried over vacuum to obtain empagliflozin as a residue.
Example 7: Preparation of Empagliflozin
[0291]The residue of compound of Formula III was dissolved in dimethylsulphoxide (210 ml) at 25-35° C. Cesium carbonate (74.5 gr) and R-Tosyl tetrahydrofuran (26.8 gr) was added. Raised the temperature of the reaction mass to 45° C. to 50° C
example 7
on of Empagliflozin-DL-Pipecolic Acid
[0292]The residue of empagliflozin, obtained according to Example 5 was added to DL-pipecolic acid (24 gr) and water (21 mL) at 80-85° C. and stirred the reaction mass for about 1 hr at 80-85° C. The reaction mass was allowed to cool to 25-35° C. and stirred for 24-48 hrs. Filtered the reaction mass and washed with n-butanol and dried the product at 45-50° C. under vacuum for 6-8 hrs.
Yield: 50 gms. HPLC: NLT 99.0%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap