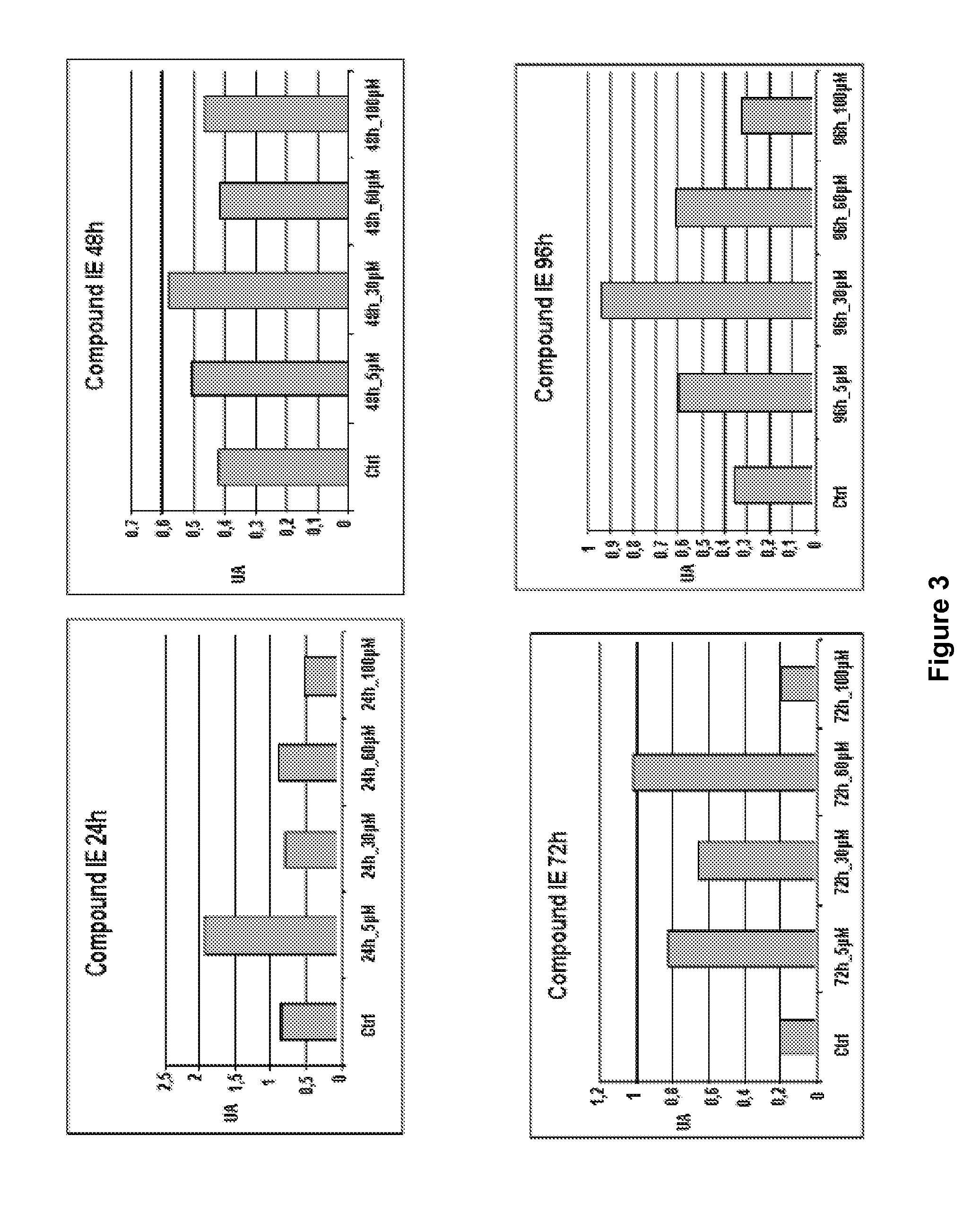
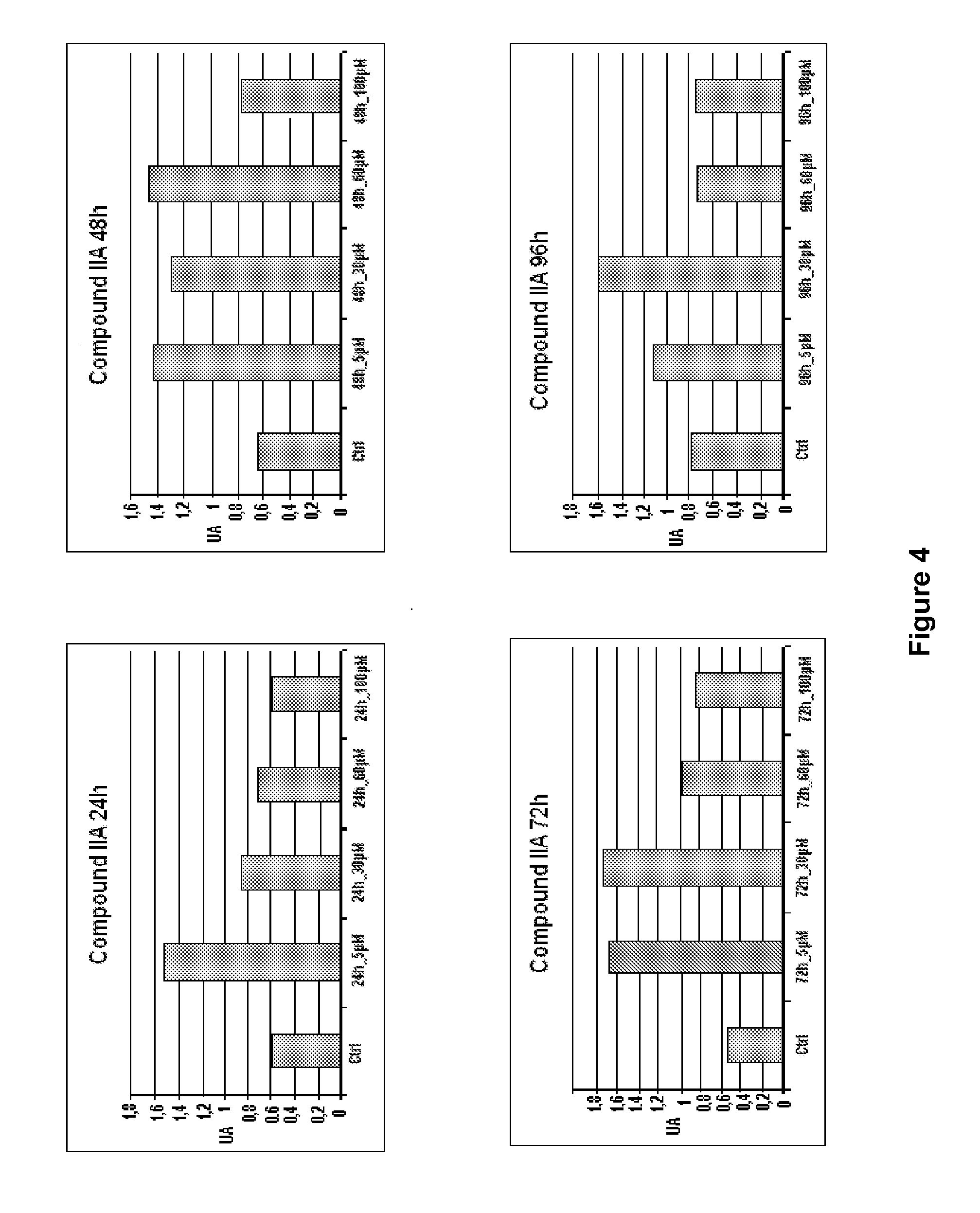
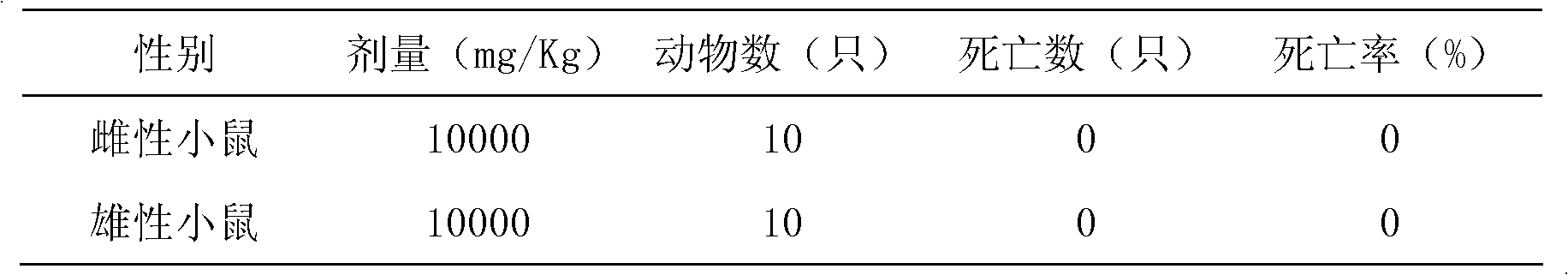
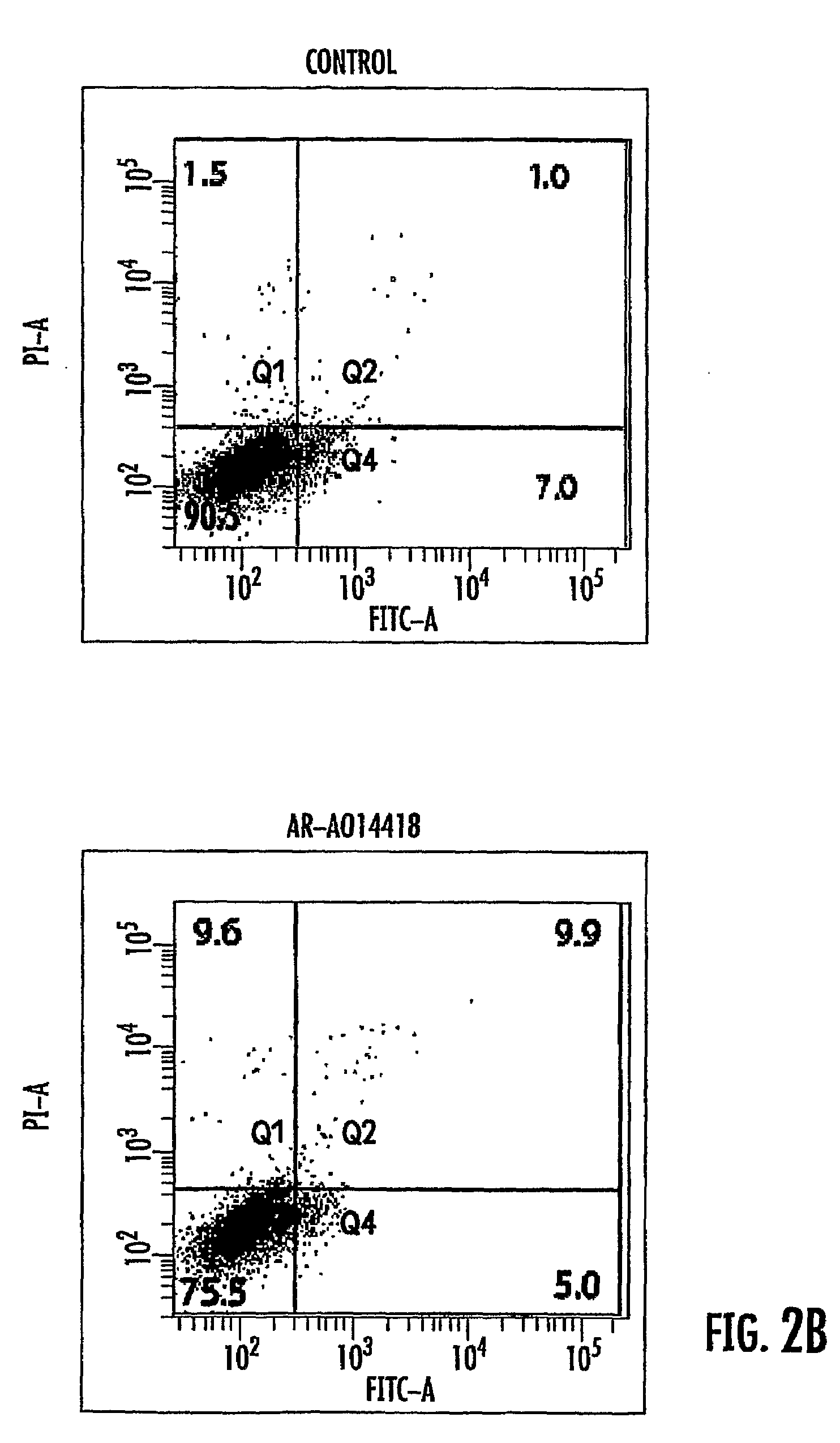
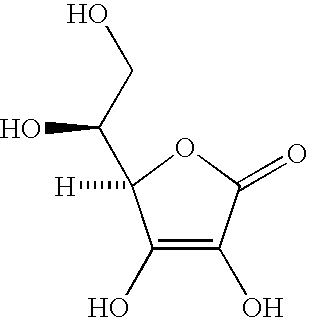
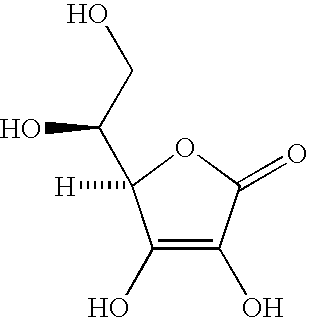
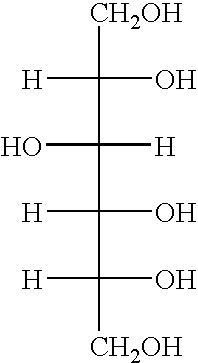
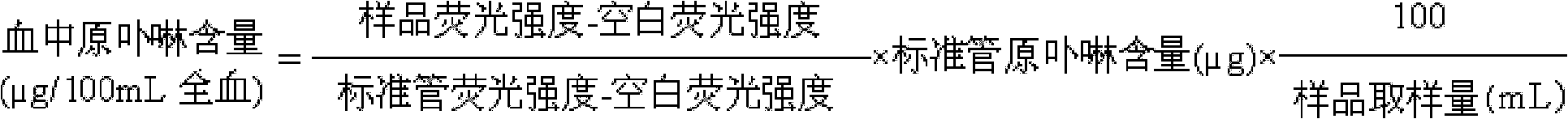Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69results about "Blood disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gastric retention controlled drug delivery system
ActiveUS20040180088A1Maintain physical integrityFast swellingOrganic active ingredientsNervous disorderControlled drugsControl release
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Bispecific Anti-vegf/Anti-ang-2 antibodies
Owner:F HOFFMANN LA ROCHE INC
Beneficial effects of increasing local blood flow
InactiveUS20110028548A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineNitric oxide
Owner:STRATEGIC SCI & TECH
Use Of Dipyridamole For Treatment Of Resistance To Platelet Inhibitors
InactiveUS20090048173A1Reduce decreaseBiocidePeptide/protein ingredientsDipyridamolePlatelet inhibitor
Owner:EISERT WOLFGANG +1
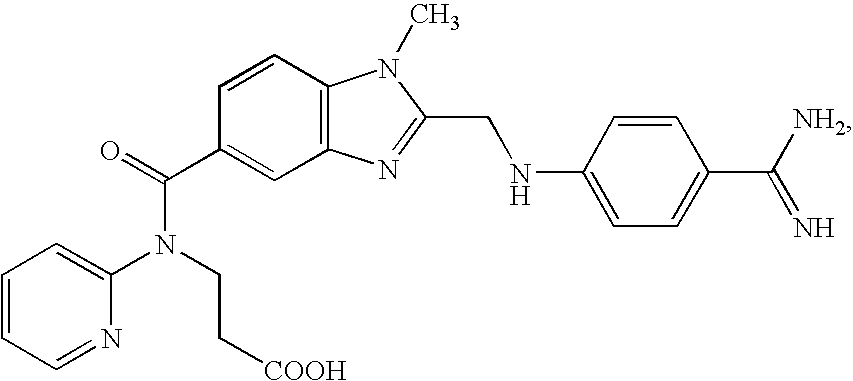
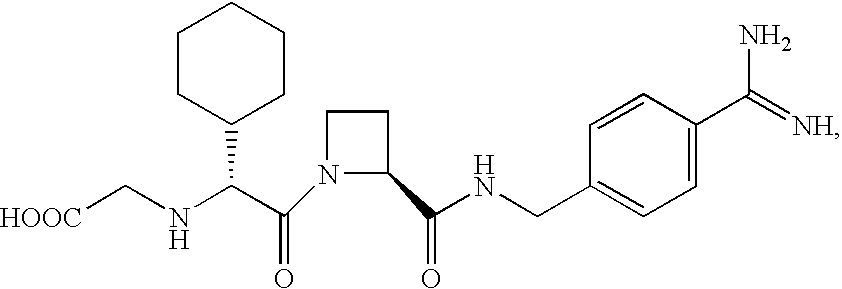
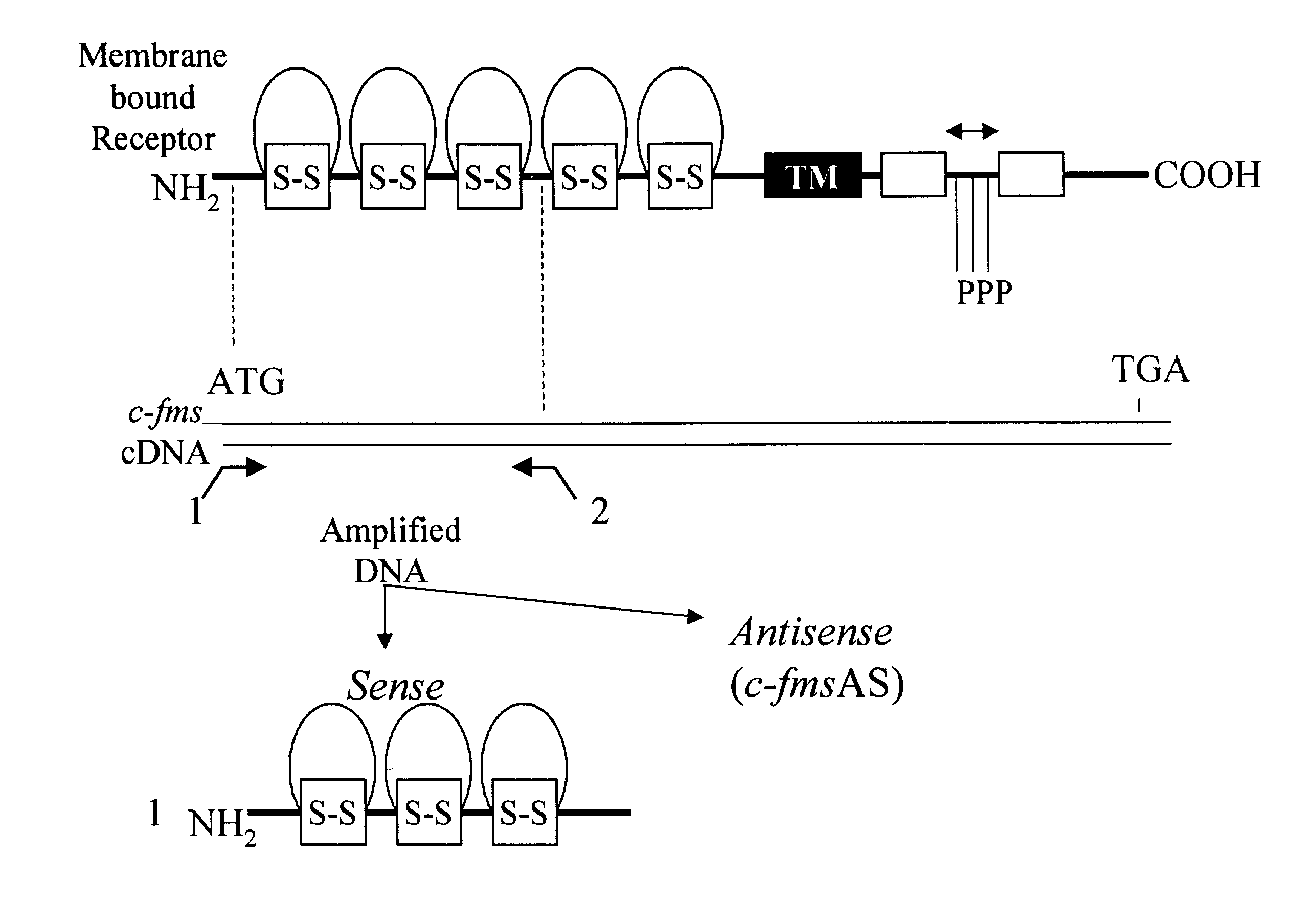
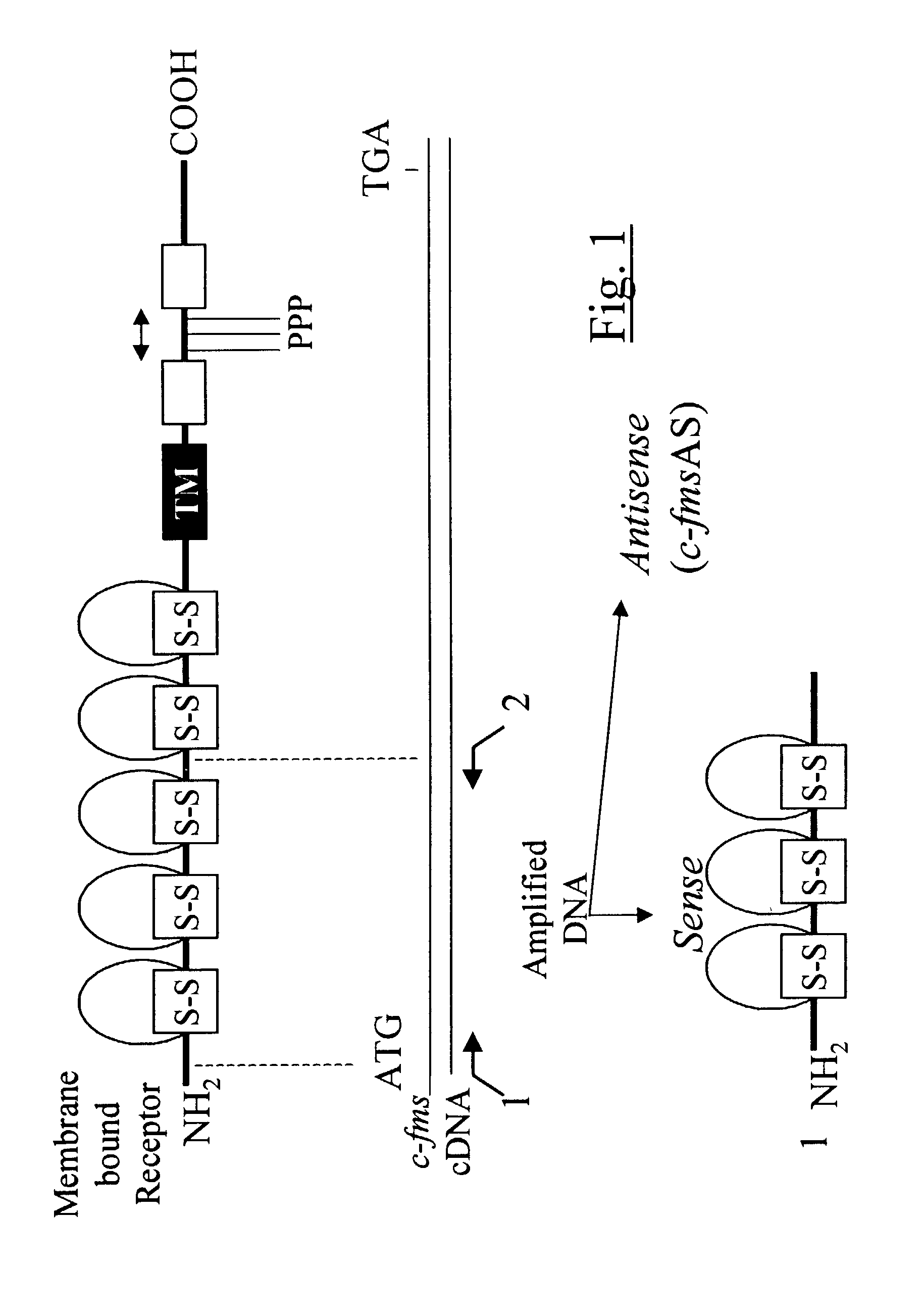
Methods for inhibiting macrophage colony stimulating factor and c-FMS-dependent cell signaling
Owner:RAJAVASHISTH TRIPATHI
Crystalline forms and process for preparing spiro-hydantoin compounds
Owner:BRISTOL MYERS SQUIBB CO
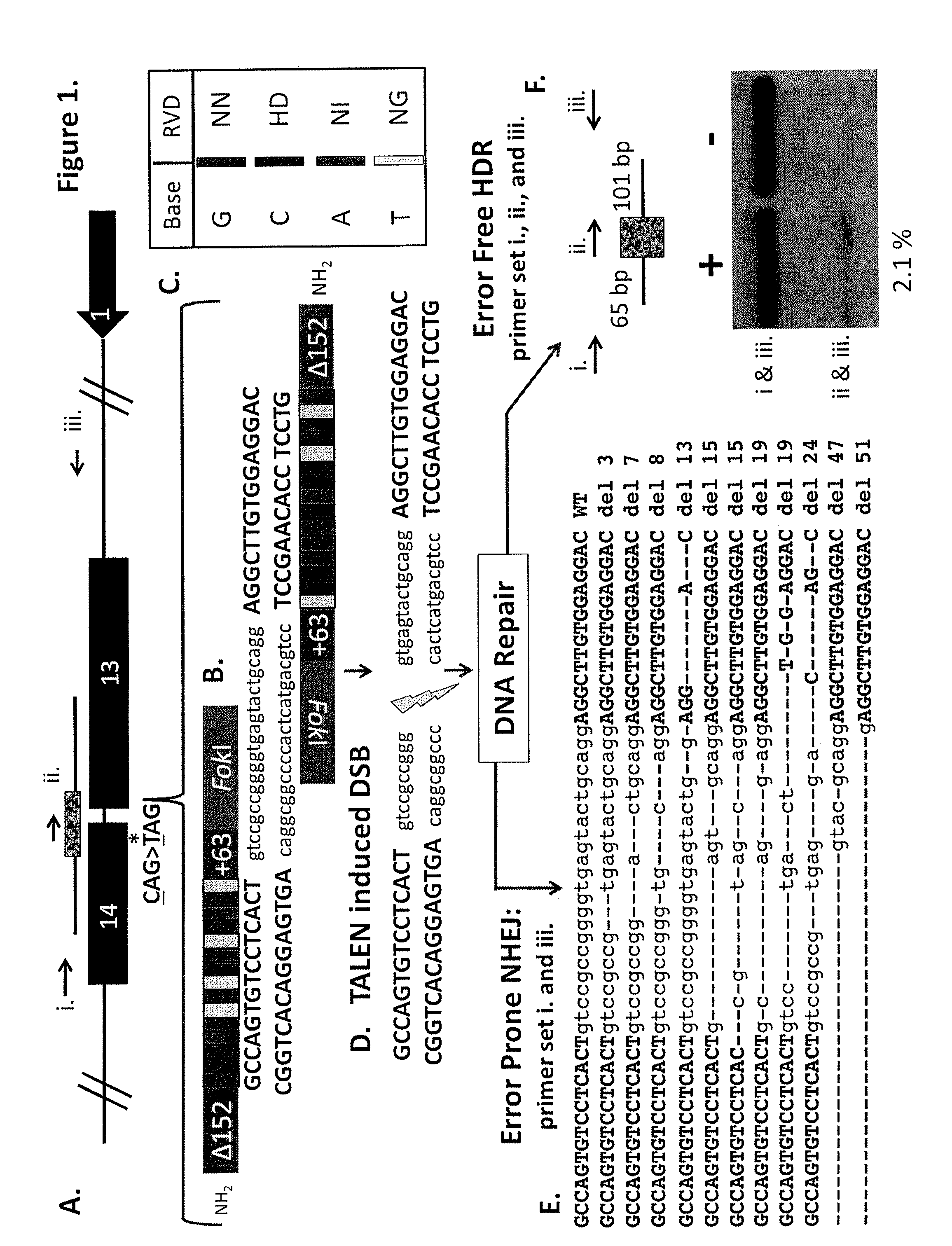
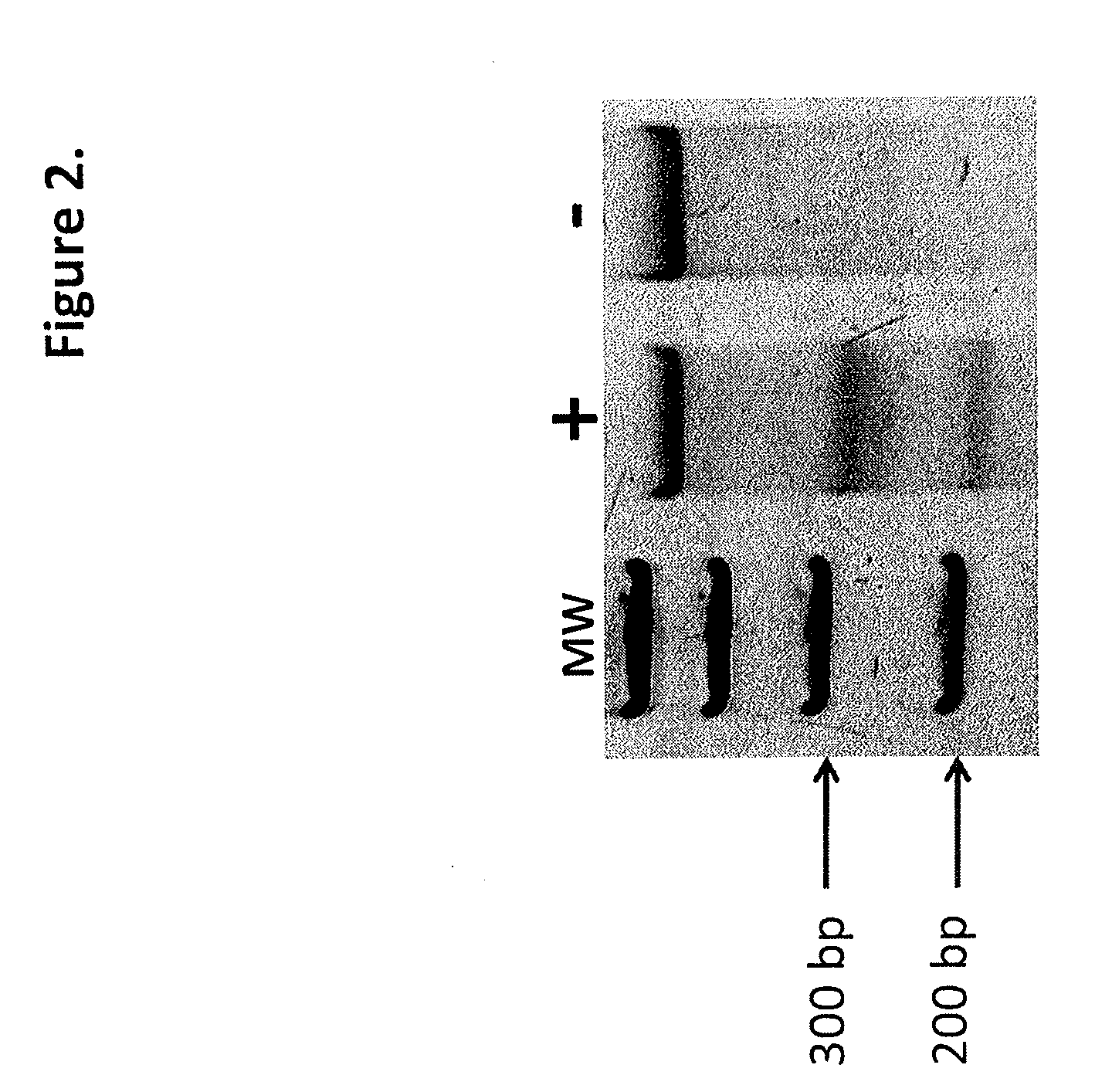
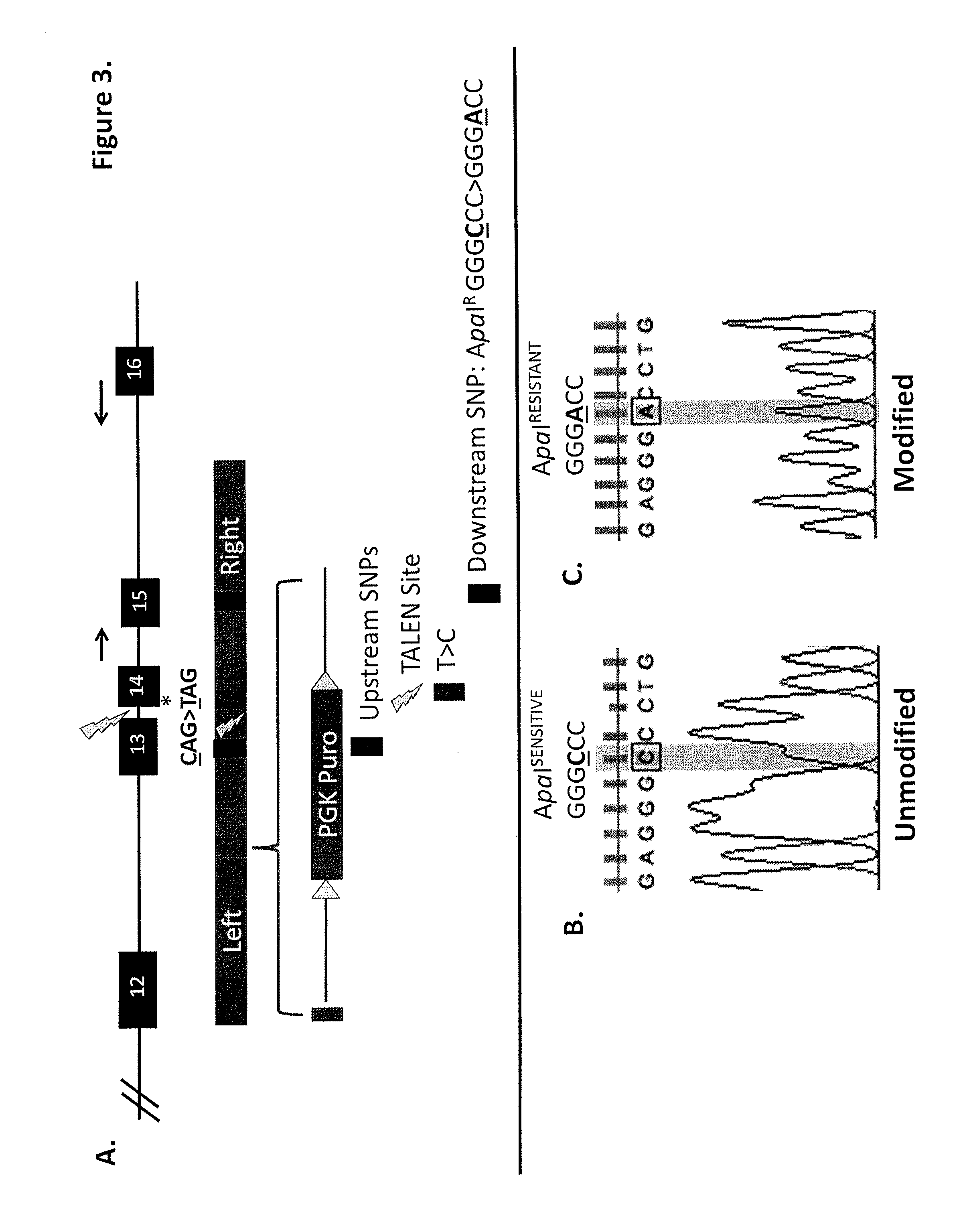
Talen-based gene correction
ActiveUS20140256798A1Restores correct gene expressionOrganic active ingredientsHydrolasesHuman DNA sequencingHuman cell
Owner:RGT UNIV OF MINNESOTA
Medicine for trenting flooding and spotting, haematemesis and homafecia
A Chinese medicine in the form of capsule, tablet, powder, dripping pill, or aerosol for treating uterine bleeding, hematemesis and hematochezia is prepared from 11 Chinese-medicinal materials including rhubarb, coptis root, notoginseng, donkey-hide gelatin, etc.
Owner:XIAN CHIHO PHARMA
Clinic compliant method for banking human placental mesenchymal cells
Owner:AFFILIATED HOSPITAL OF NINGXIA MEDICAL UNIV
USE OF PHTHALIMIDE AND/OR SULPHONAMIDE DERIVATIVES IN THE TREATMENT OF DISEASES WHICH REQUIRE REDUCING THE TNF-alpha LEVELS AND AN EXOGENOUS SOURCE OF NITRIC OXIDE, PHTHALIMIDE DERIVATIVES, SULPHONAMIDE DERIVATIVES, AND A METHOD FOR OBTAINING A SULPHONAMIDE DERIVATIVE
InactiveUS20100324107A1Improve the quality of lifeBiocideOrganic chemistryPhthalocyanine derivativesNitric oxide
Owner:UNIV ESTADUAL DE CAMPINAS UNICAMP +2
Traditional Chinese medicine health care product, its preparation method and its application
ActiveCN102552335AImprove sleepingImprove anemiaNervous disorderAnthropod material medical ingredientsSide effectCordyceps cicadae
Owner:ZHEJIANG BIOASIA PHARMA CO LTD
Fibrin-Binding Peptides and Conjugates Thereof
ActiveUS20100158814A1High degreeSuperior fibrin specific bindingUltrasonic/sonic/infrasonic diagnosticsCompound screeningBinding peptideCompanion animal
Owner:BRACCO IMAGINIG SPA
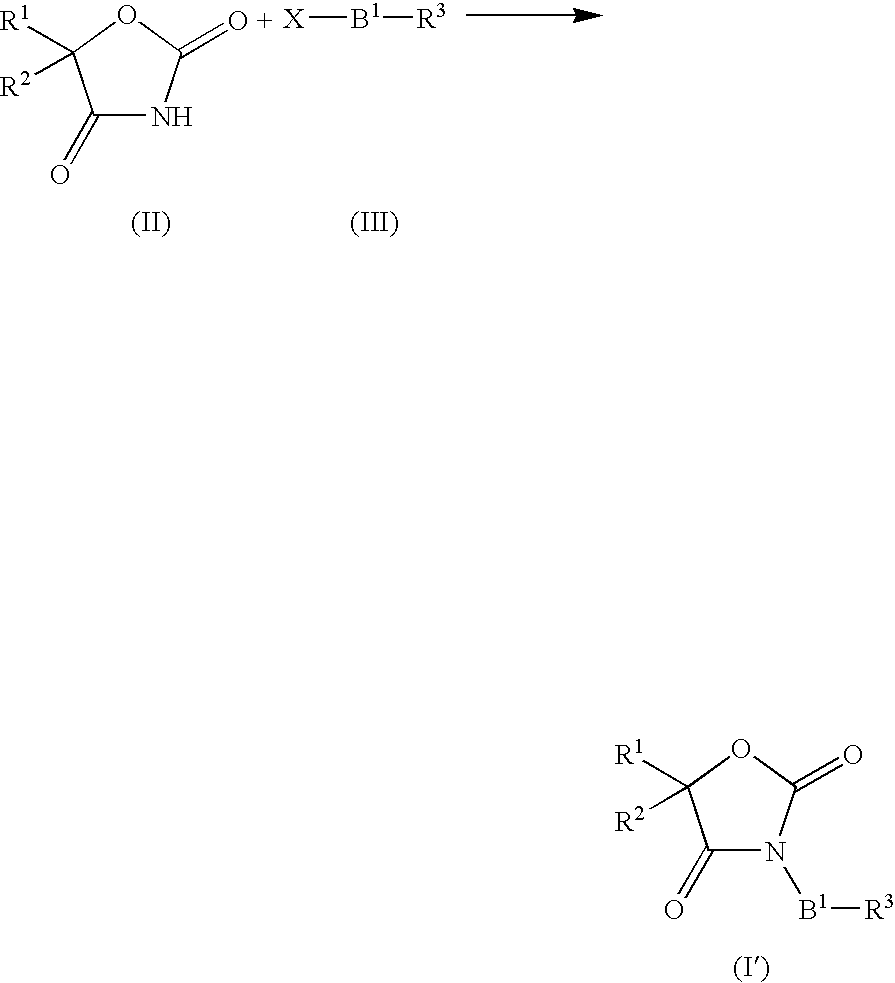
Novel imidazolidinedione derivatives and use thereof as drugs
Owner:SENJU PHARMA CO LTD
Peptide for inhibiting dipeptidyl-peptidase iv
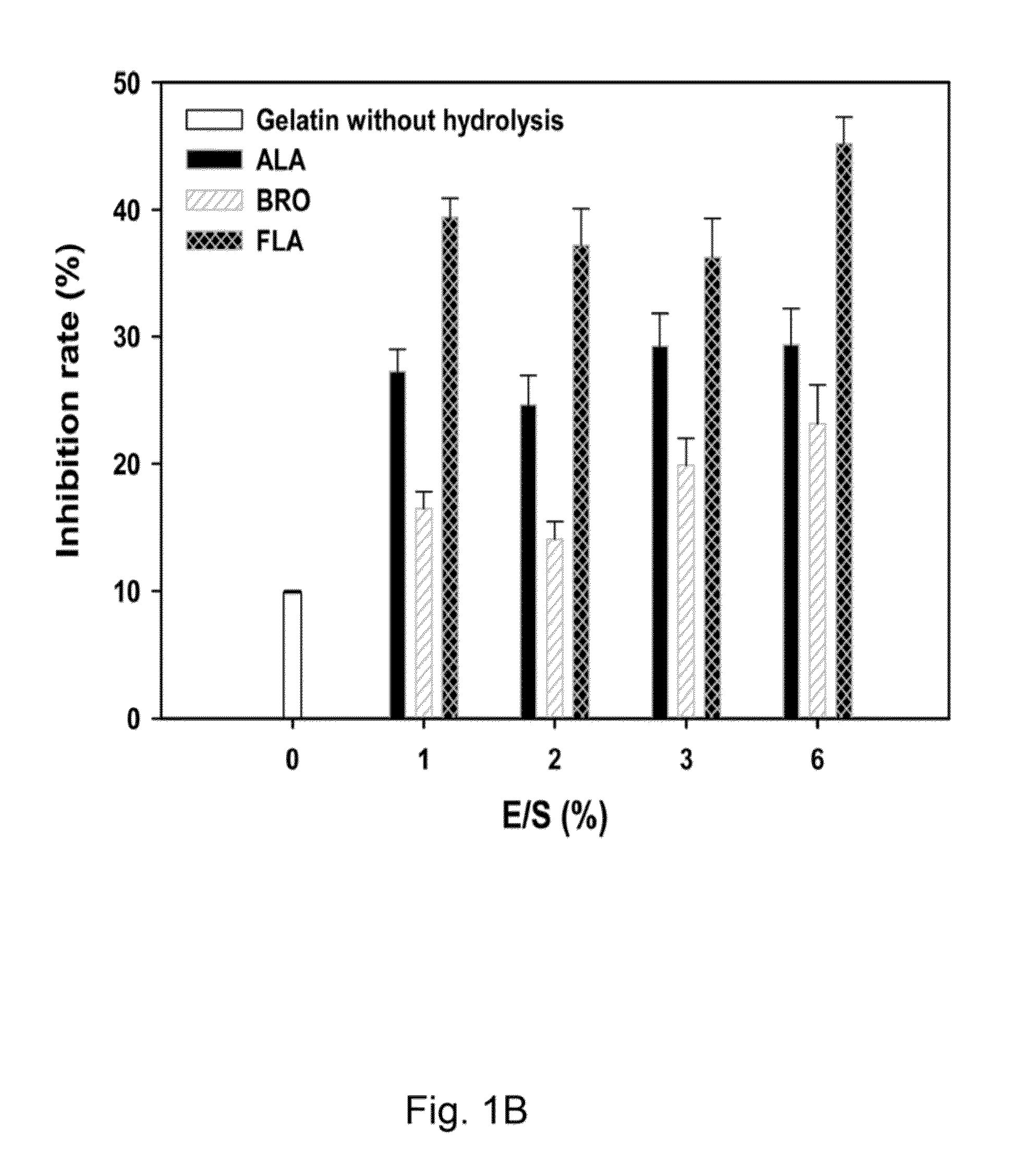
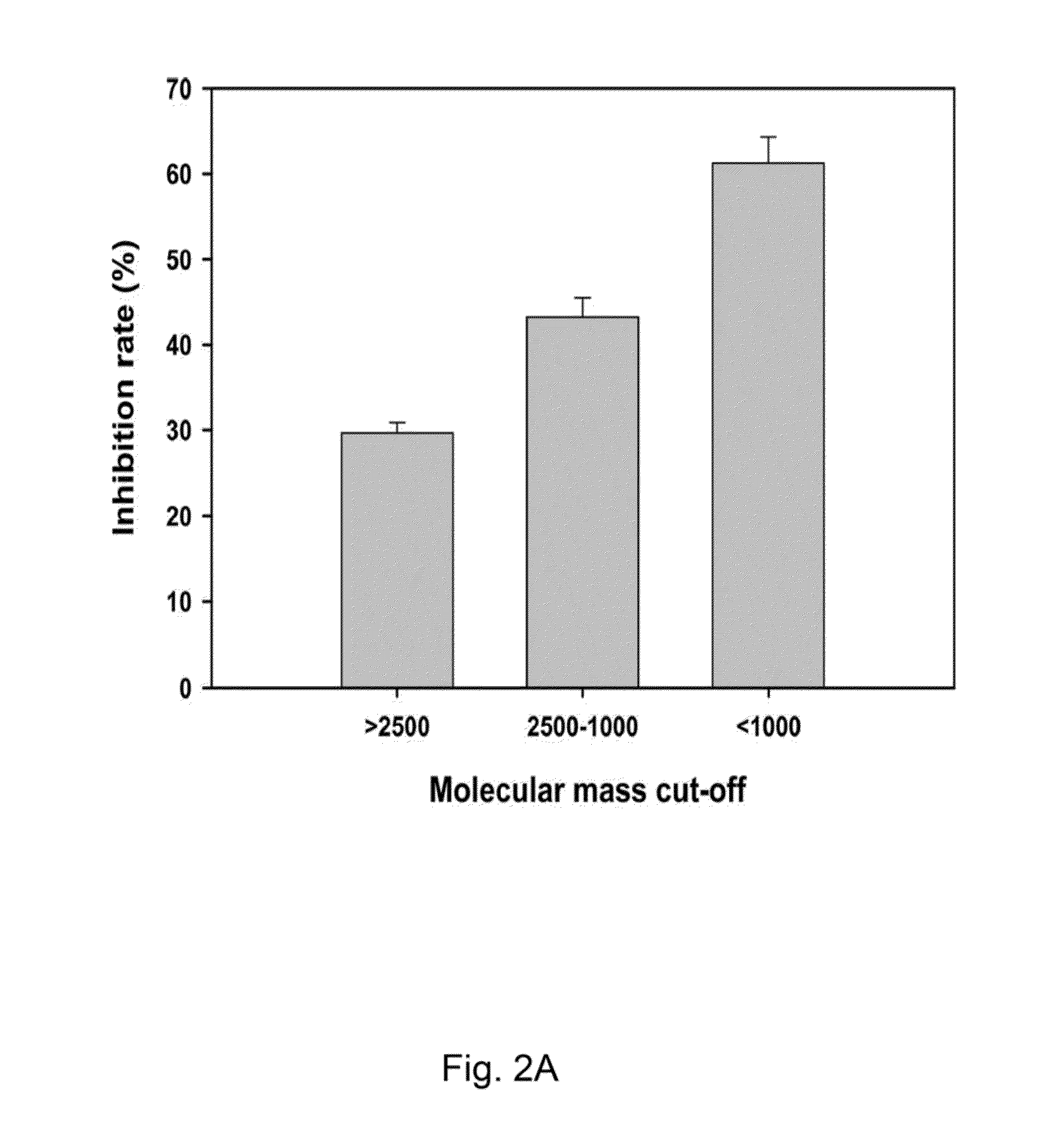
InactiveUS20140193463A1Metabolism disorderTetrapeptide ingredientsGelatin hydrolysateEnzymatic digestion
Owner:CHINA MEDICAL UNIVERSITY(TW)
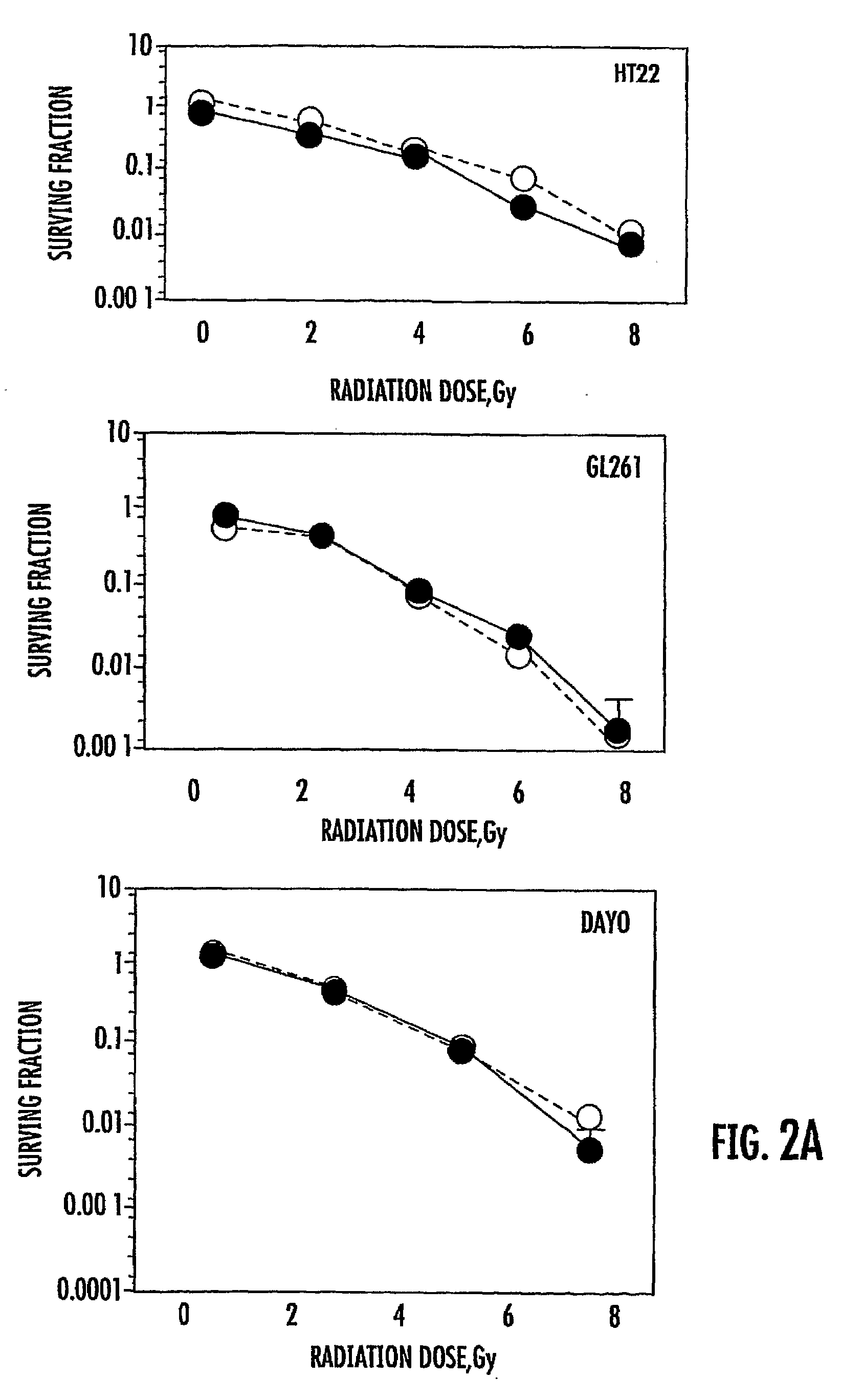
Use of GSK3 inhibitors in combination with radiation therapies
Owner:VANDERBILT UNIV
Chinese patent medicine for treating idiopathic edema
InactiveCN102366617AEdema disappearsReduce puffinessBlood disorderPlant ingredientsDiseaseSide effect
The invention provides a Chinese patent medicine for treating idiopathic edema, which comprises the following components by weight part: 9-15 parts of tribulus terrestris, 8-12 parts of semen psoraleae, 6-10 parts of radix angelicae, 4-8 parts of placenta hominis, 4-8 parts of fleece flower root, 3-5 parts of Maytenus hookeri, 1-3 parts of gen-seng and 1-3 parts of spatholobus stem. Compared with the prior art, the Chinese patent medicine of the present invention aims at internal disease of idiopathic edema, is capable of performing therapies with syndrome differentiation, and has the advantages of fast curative effect, low cost, no toxicity, no side-effect and substantial effect. The Chinese patent medicine for treating idiopathic edema treats both principal and secondary aspect of disease.
Owner:CHENGDU LYUDI TECH
Nutrition wine for nourishing yin and invigorating kidney
InactiveCN102586064ANourishing yin and yangWith blood circulationDigestive systemAlcoholic beverage preparationBiotechnologySide effect
Owner:张新华
Stable ascorbic acid compositions
InactiveUS20070077220A1Improve stabilitySatisfactory shelf lifeBiocideCosmetic preparationsVitamin CAlcohol
Owner:OMP
Cold storage of modified platelets
InactiveUS20080193430A1Acceptable platelet functionalityAcceptable viabilityBiocideMammal material medical ingredientsPolyethylene glycolPlatelet storage
Owner:CANADIAN BLOOD SERVICES
Hemostatic products
A method of inducing hemostasis in a wound. A hemostatic product is applied to a wound. The hemostatic product includes at least one hemostasis component. The hemostatic product is retained with respect to the wound by positioning a hydrogel material at least partially over the hemostatic product. At least a portion of the hemostatic product is dissolved. Hemostasis is induced in the wound with the at least one hemostasis component. The hydrogel material is separated from the wound. Substantially all of the hemostatic product remains on the wound.
Owner:ST TERESA MEDICAL
Plant source alcohol soluble protein and its preparation method
InactiveCN1634613APro-proliferativePromote generationPharmaceutical containersSurgeryAlcoholSeparation technology
Owner:SHANGHAI JIAO TONG UNIV
Preparation method of sodium ibandronate
InactiveCN102898466AEasy to operateMeet the quality requirements of raw materialsGroup 5/15 element organic compoundsSkeletal disorderPhosphorous acidChlorobenzene
The invention relates to the technical field of pharmaceutical chemistry, particularly relates to a method of pharmaceutical synthesis, and specifically relates to a preparation method of sodium ibandronate. To overcome the disadvantages of high content of chlorides and phosphites in sodium ibandronate prepared by a conventional preparation method of sodium ibandronate, the preparation method of sodium ibandronate with extremely low content of chlorides and phosphites is provided. In the preparation method, 3-(N-methylpentylamino) propionic acid hydrochloride, phosphorus trichloride and phosphorous acid are employed as raw materials and reacted in a chlorobenzene solvent, so as to obtain sodium ibandronate with extremely low content of the chlorides and the phosphites. The obtained sodium ibandronate can not only meet impurity control standards of the chlorides and the phosphites in a sodium ibandronate crude drug, but also prevent low yield, long period and huge harm to human body and environment which are brought by a lot of refining steps.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of high-purity gynostemma pentaphylla total saponin for veterinary drug
ActiveCN103520256AHigh purityMeet the protection requirementsAntibacterial agentsAntimycoticsReflux extractionMicrofiltration
Owner:江西嘉博生物工程有限公司 +1
Traditional Chinese medicine for treating anemia
InactiveCN101966283AImprove the effect of adjuvant therapyGood treatment effectUnknown materialsBlood disorderTreatment effectScrophularia
Owner:王风菊
Traditional Chinese medicine formula for improving hematopoietic function of female animals and young animals and preparation method thereof
InactiveCN111388565APromote growthImprove immunityHeavy metal active ingredientsOrganic active ingredientsBiotechnologyDisease
The invention discloses a traditional Chinese medicine formula for improving hematopoietic function of female animals and young animals and a preparation method thereof. The formula is used for improving the hematopoietic function of the female animals and the young animals, and belongs to the technical field of veterinary drugs. The traditional Chinese medicine formula comprises the following rawmaterial components in parts by weight: 20-30 parts of gas-explosion traditional Chinese medicine powder, 20-30 parts of ferric glycinate and 30-50 parts of baked soybean powder. The traditional Chinese medicine formula provided by the invention has the advantages of rapid drug effect, high content of effective components and rapid release, and can play a good role in tonifying qi and activatingblood, preventing and treating anemia, and improving body immunity and disease resistance.
Owner:KAIFENG JIAJUN BIOLOGICAL SCI & TECH CO LTD
Glucopyranosyloxypyrazole derivatives and medicinal use thereof
InactiveUS20060142209A1Easy to optimizeEnhancing glucose uptakeBiocideAntibiotics chemistryAcute hyperglycaemiaHydrogen atom
Owner:KISSEI PHARMA
COMPOSITION FOR REPROGRAMMING SOMATIC CELLS TO GENERATE INDUCED PLURIPOTENT STEM CELLS, COMPRISING Oct4 IN COMBINATION WITH Bmi1 OR ITS UPSTREAM REGULATOR, AND METHOD FOR GENERATING INDUCED PLURIPOTENT STEM CELLS USING THE SAME
Owner:KOREA UNIV RES & BUSINESS FOUND
Method for preparing medicament for treating leucopenia
InactiveCN103055036ATo promote metabolismFunction increaseUnknown materialsBlood disorderReflux extractionLiver and kidney
The invention relates to a method for preparing a medicament for treating leucopenia. The preparation method comprises the following steps of: taking medicinal materials in a ratio, adding 60 to 80 percent ethanol in an amount which is 6 to 10 times the weight of the medicinal materials, performing reflux extraction for 2 to 4 times and 1 to 3 hours every time, combining the ethanol extracts, and recovering the ethanol till the ethanol smell does not exist to obtain paste; decocting the medicinal residue for 2 to 4 hours and 1 to 3 hours every time by adding water in an amount which is 4 to 6 times the weight of the medicinal residue, combining the decoction, and concentrating; and combining the medicinal paste, concentrating the medicinal paste into thick paste under reduced pressure, adding available medicinal accessories into the thick paste, thus obtaining a clinical available preparation. The medicament has the effects of nourishing liver and kidney and tonifying qi and blood, and is clinically used for treating the leucopenia produced after malignant tumor radiotherapy and chemotherapy.
Owner:朱家红
Brewing method of agaricus bisporus and chenopodium quinata wine
InactiveCN107746765AImprove immunityEnhance hypoglycemiaMetabolism disorderMicroorganism based processesAdditive ingredientGenus Chenopodium
Owner:JIANGSU HENGSHUN VINEGAR IND
Cassia twig and poria cocos pills and preparation method thereof
InactiveCN106177026ALow costSmall side effectsPharmaceutical non-active ingredientsPill deliverySide effectRemove blood
Owner:上海典实医疗科技有限公司
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap