Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58results about "Pill delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
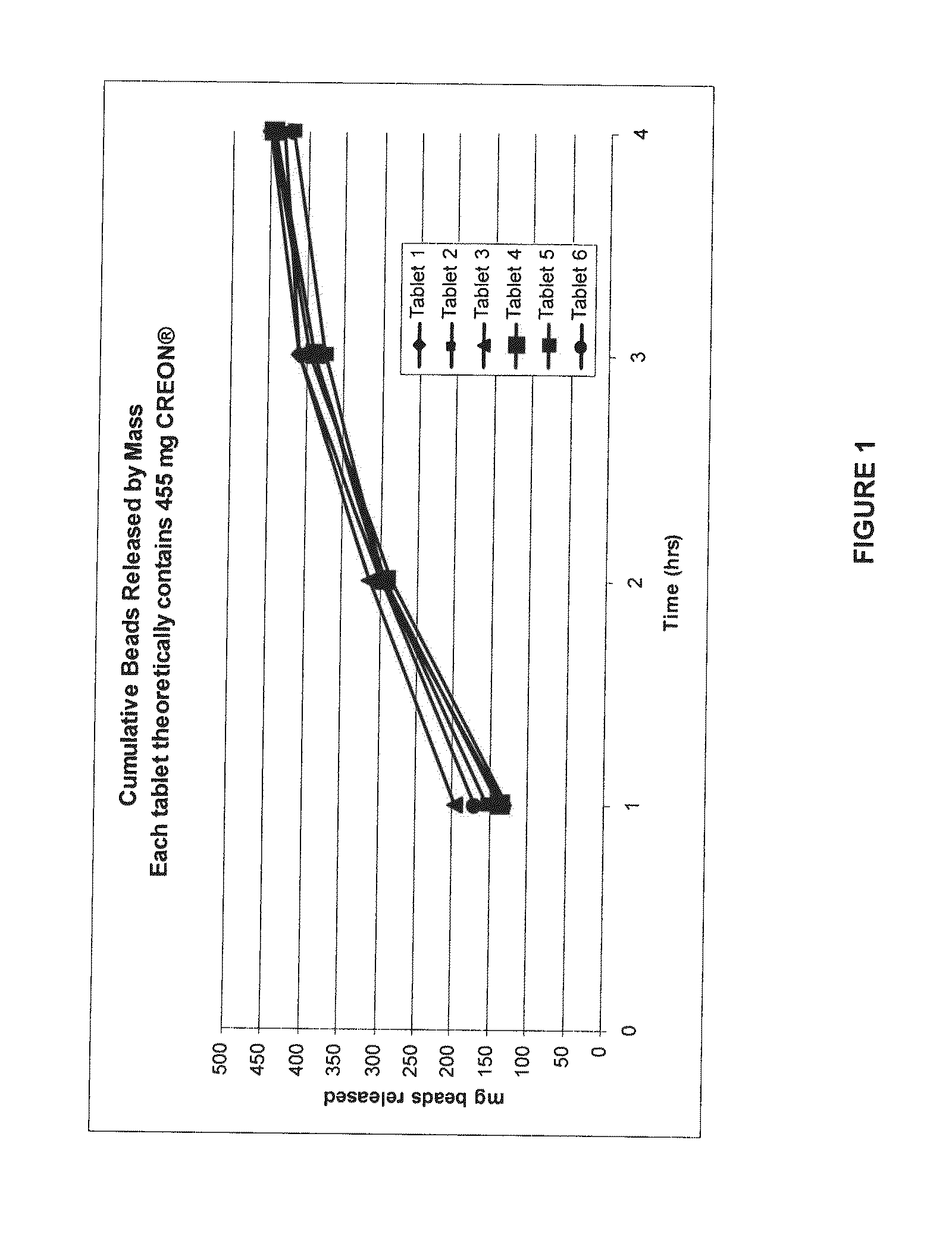
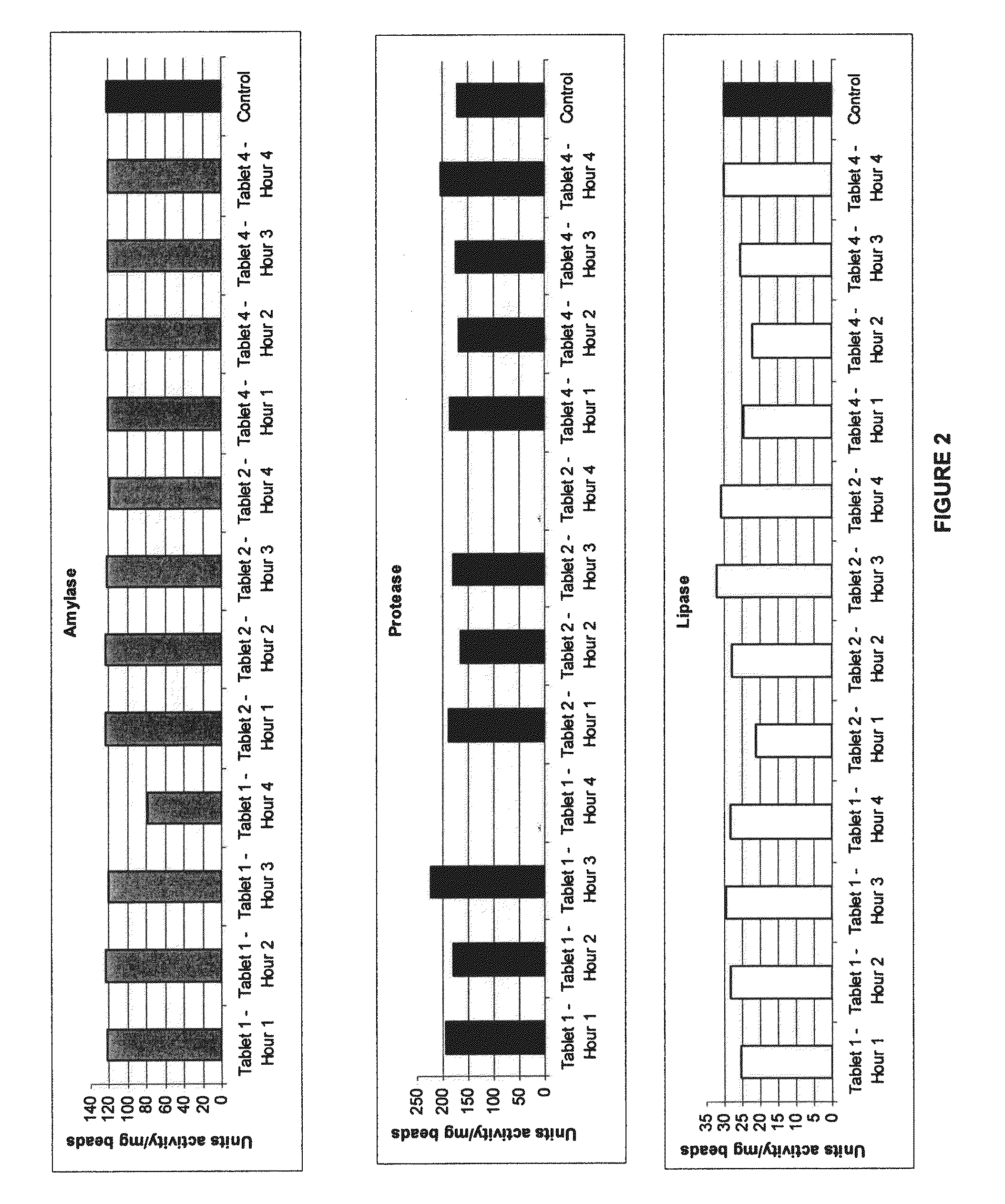
Gastric retention controlled drug delivery system
ActiveUS20040180088A1Maintain physical integrityFast swellingOrganic active ingredientsNervous disorderControlled drugsControl release
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Controlled-release formulations, method of manufacture, and use thereof
Owner:SUN PHARMA IND INC
Health-care food with functions of improving immunocompetence and protecting damaged gastric mucosa and its preparing method
Owner:吉林云尚保健食品有限公司
Medicine for trenting flooding and spotting, haematemesis and homafecia
A Chinese medicine in the form of capsule, tablet, powder, dripping pill, or aerosol for treating uterine bleeding, hematemesis and hematochezia is prepared from 11 Chinese-medicinal materials including rhubarb, coptis root, notoginseng, donkey-hide gelatin, etc.
Owner:XIAN CHIHO PHARMA
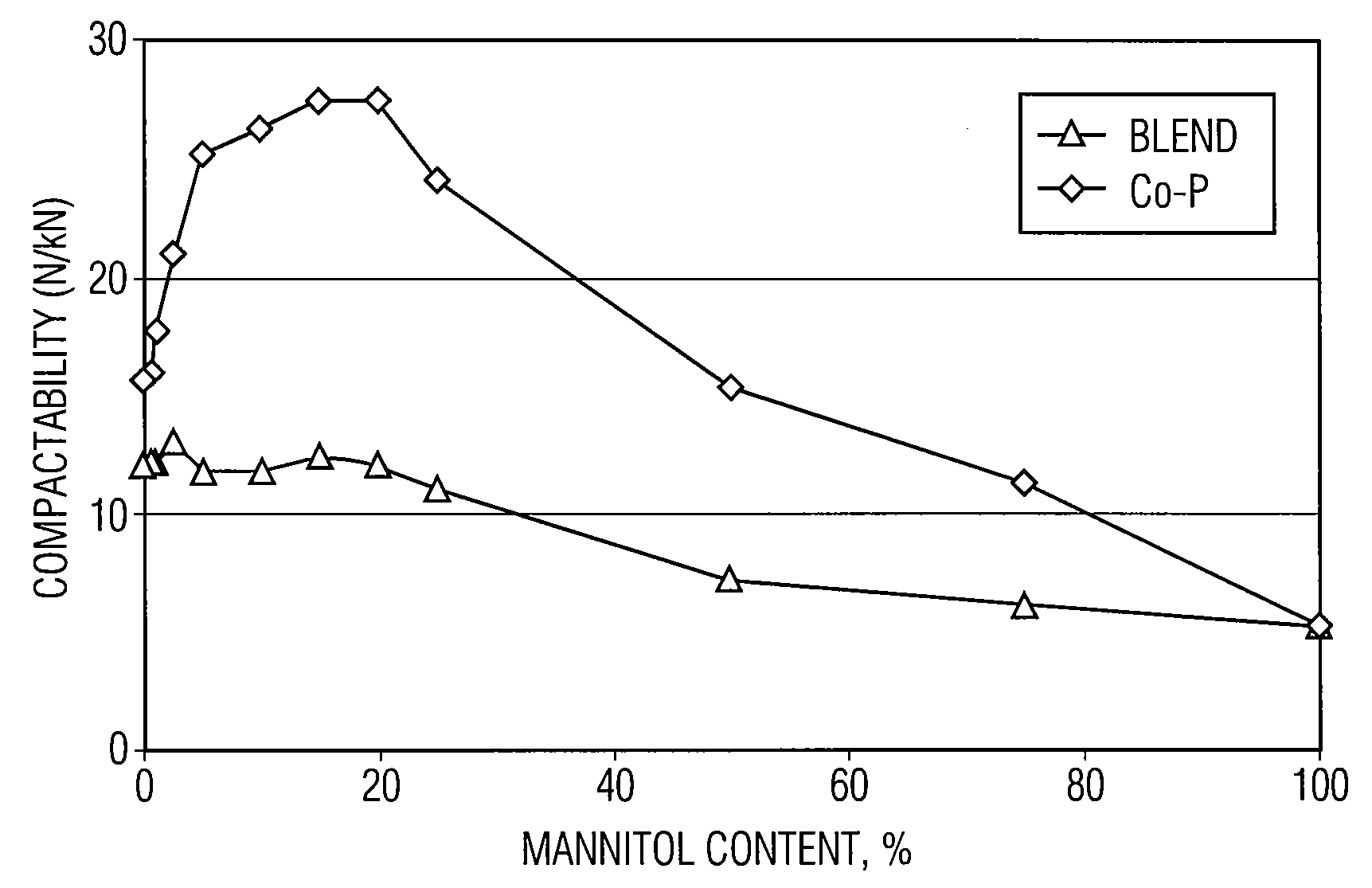
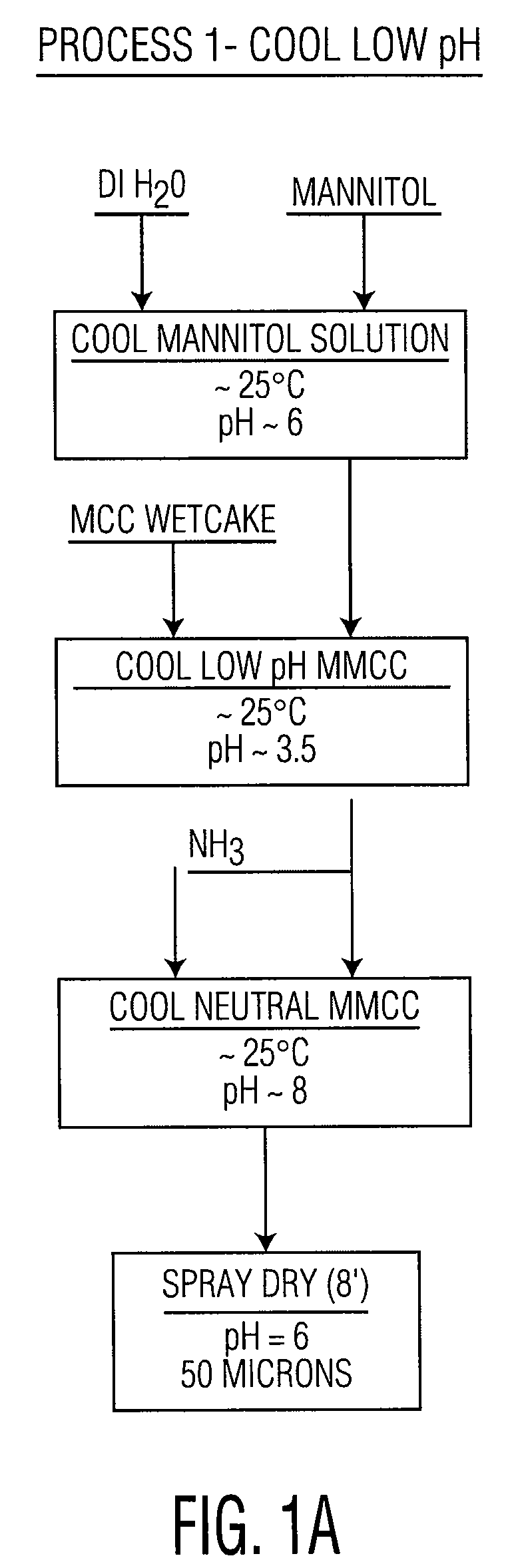
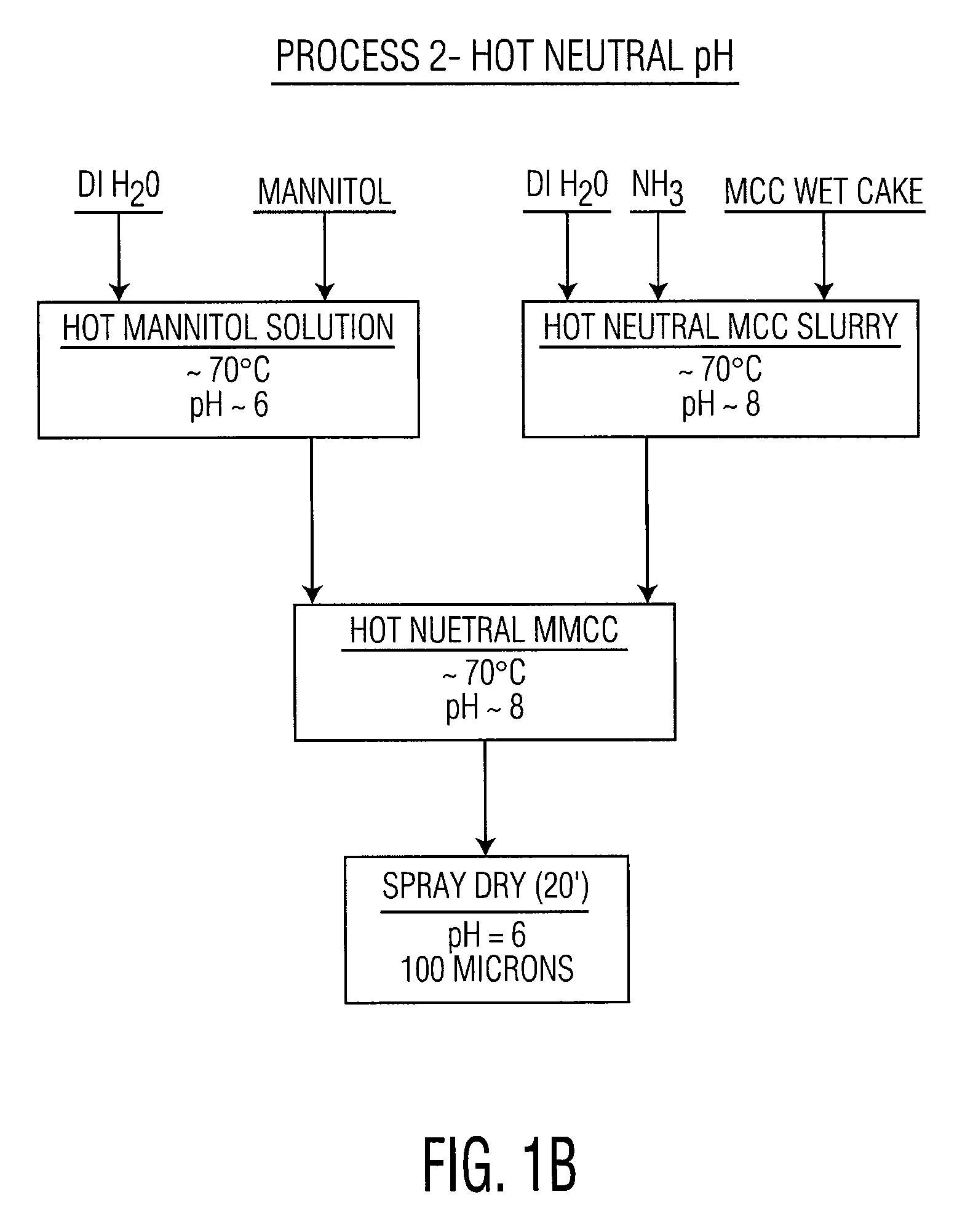
Co-processed microcrystalline cellulose and sugar alcohol as an excipient for tablet formulations
ActiveUS20080131505A1Improve the compaction effectWood working apparatusPill deliveryParticulatesMANNITOL/SORBITOL
Owner:DUPONT NUTRITION USA INC
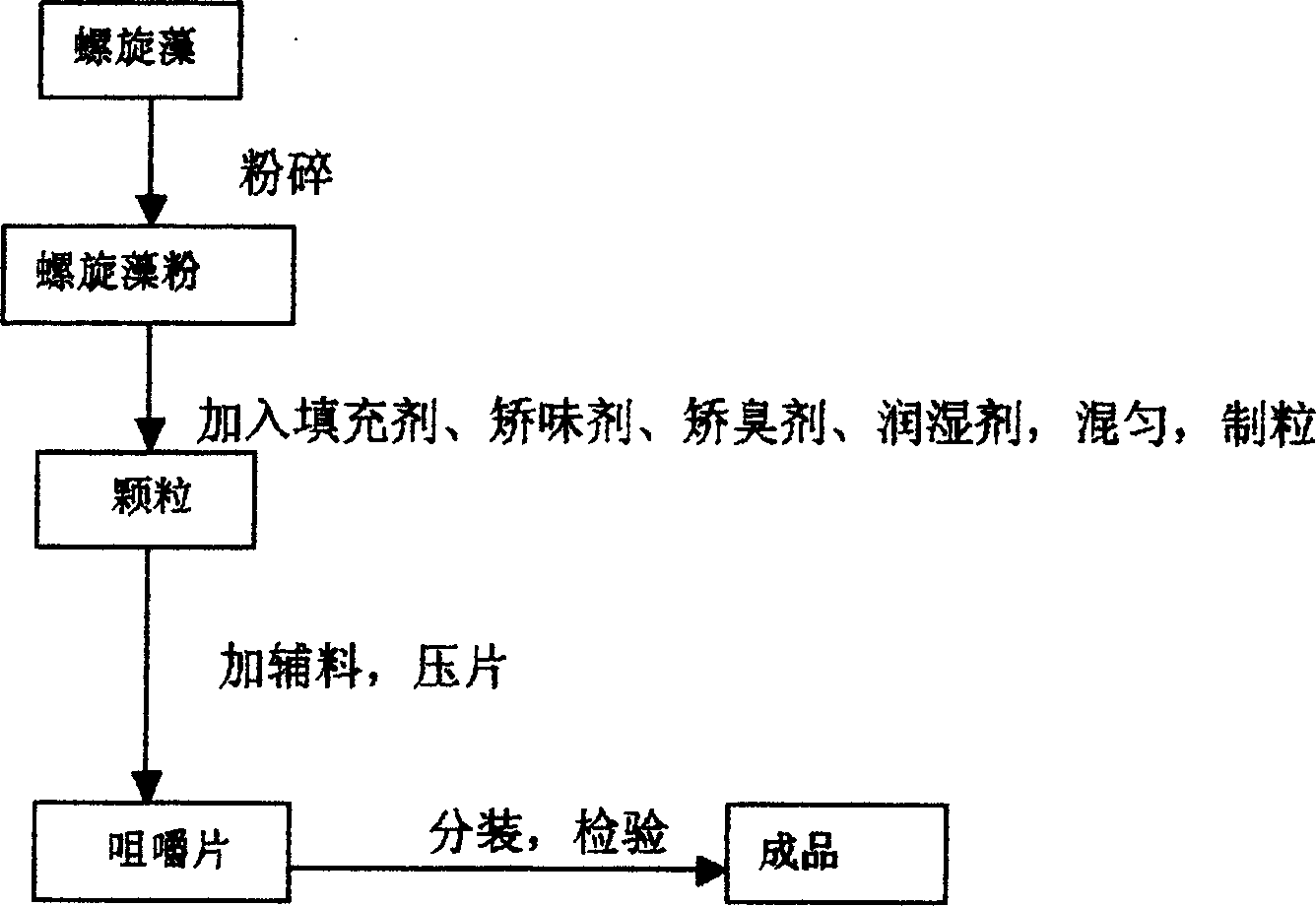
Spirulina chewing tablets and prepn. thereof
InactiveCN1833661AEasy to acceptEasy to takeMetabolism disorderAlgae medical ingredientsMANNITOL/SORBITOLAlcohol
Owner:石圣洪
Mucosal Bioadhesive SLow Release Carrier for Delivering Active Principles
A mucosal bioadhesive slow release carrier comprising an active principle and devoid of starch, lactose, which can release the active principal for a duration of longer than 20 hours. This bioadhesive carrier contains a diluent, an alkali metal alkylsulfate, a binding agent, at least one bioadhesive polymer and at least one sustained release polymer, as well as a method for its preparation.
Owner:ONXEO SA
Controlled release formulation for treating sleep disorders
The invention relates to a controlled-release formulation for preventing and / or treating sleep disorders comprising Zaleplon or a pharmaceutically acceptable salt thereof in immediate release form and Zolpidem or a pharmaceutically acceptable salt thereof in sustained release form, wherein Zaleplon or a pharmaceutically acceptable salt thereof and Zolpidem or a pharmaceutically acceptable salt thereof are released in two phases where the first phase is a immediate release phase of Zaleplon or a pharmaceutically acceptable salt thereof and the second phase is a sustained release phase of Zolpidem or a pharmaceutically acceptable salt thereof.
Owner:TAIWAN BIOTECH
Composition and method for treatment of metabolic disorders
ActiveUS20180228863A1Safely eliminated through excretionFast dissolutionDispersion deliveryPeptide/protein ingredientsTherapeutic effectEndocrine system
The present invention provides compositions and methods for treatment of metabolic syndromes. Namely, the presently disclosed compositions and methods are provided for affecting the function of the gastrointestinal endocrine system in key regions of the gut, thereby, producing therapeutic effects on obesity, diabetes and other metabolic disorders. The compositions include components for forming luminal barriers within the gastrointestinal tract of a subject where the barrier is created in-situ via interaction of resident mucin with the mucin-interacting agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
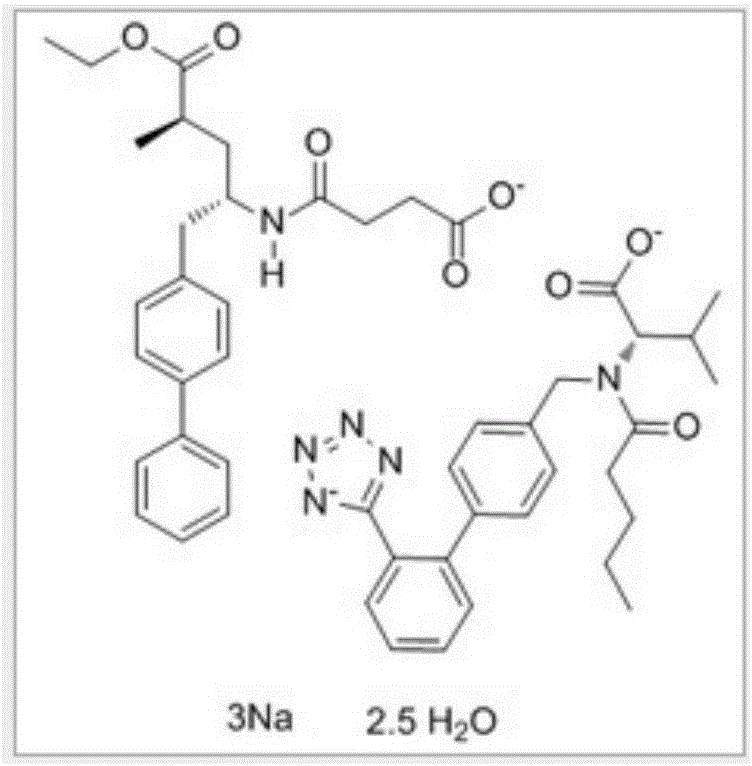
Preparation method of LCZ696 sustained release matrix tablet for treatment of heart failure
ActiveCN105748420AReduce releaseStable blood concentrationPill deliveryPharmaceutical non-active ingredientsSustained Release TabletSide effect
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Compressed tablet containing cannabidiol, method for its manufacture and use of such tablet in oral treatment of psychosis or anxiety disorders
ActiveUS20160279077A1Easy to useImprove oral bioavailabilityNervous disorderHydroxy compound active ingredientsSucroseOral treatment
Owner:ECHO PHARM BV (NL)
Gastric-dissolved and enteric-dissolved pills of traditional Chinese medicine capable of strengthening spleen and nourishing intestine and its preparation method
ActiveCN101007155AStop stomach bleedingLower purchase costHeavy metal active ingredientsDigestive systemHalloysiteIntestinal structure
Owner:GUANGZHOU CHEN LI JI PHARMA FACTORY
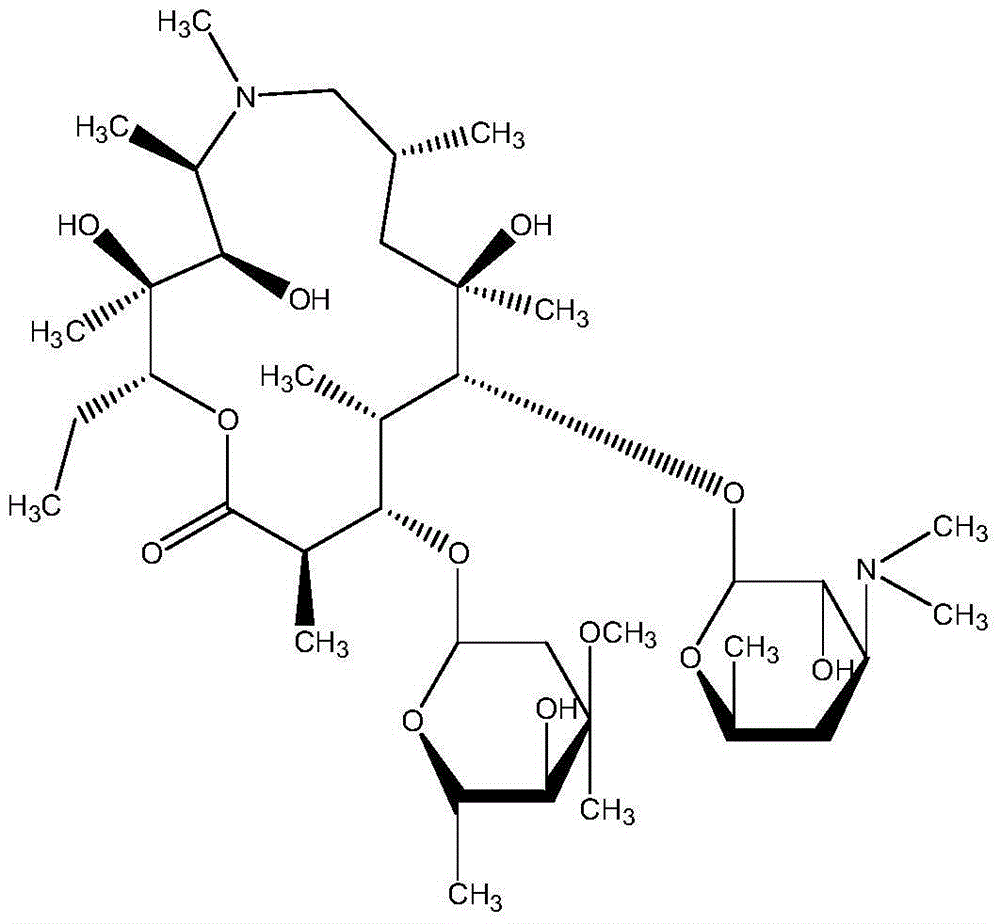
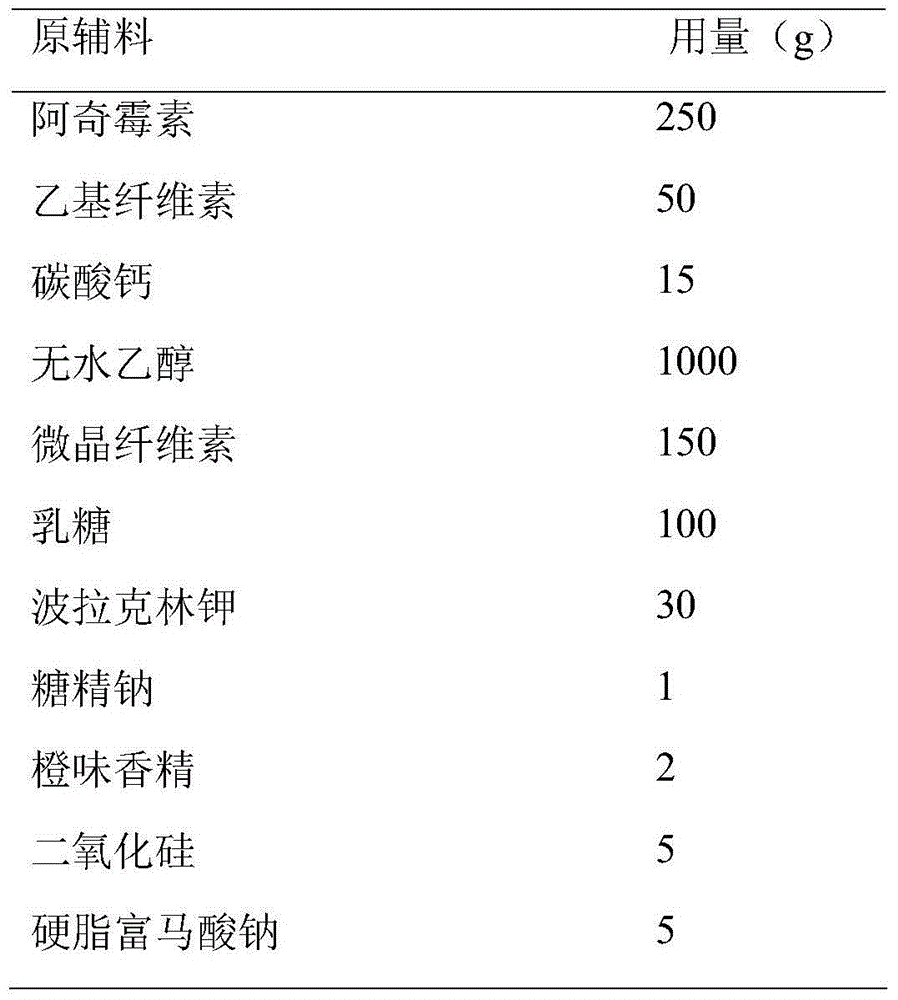
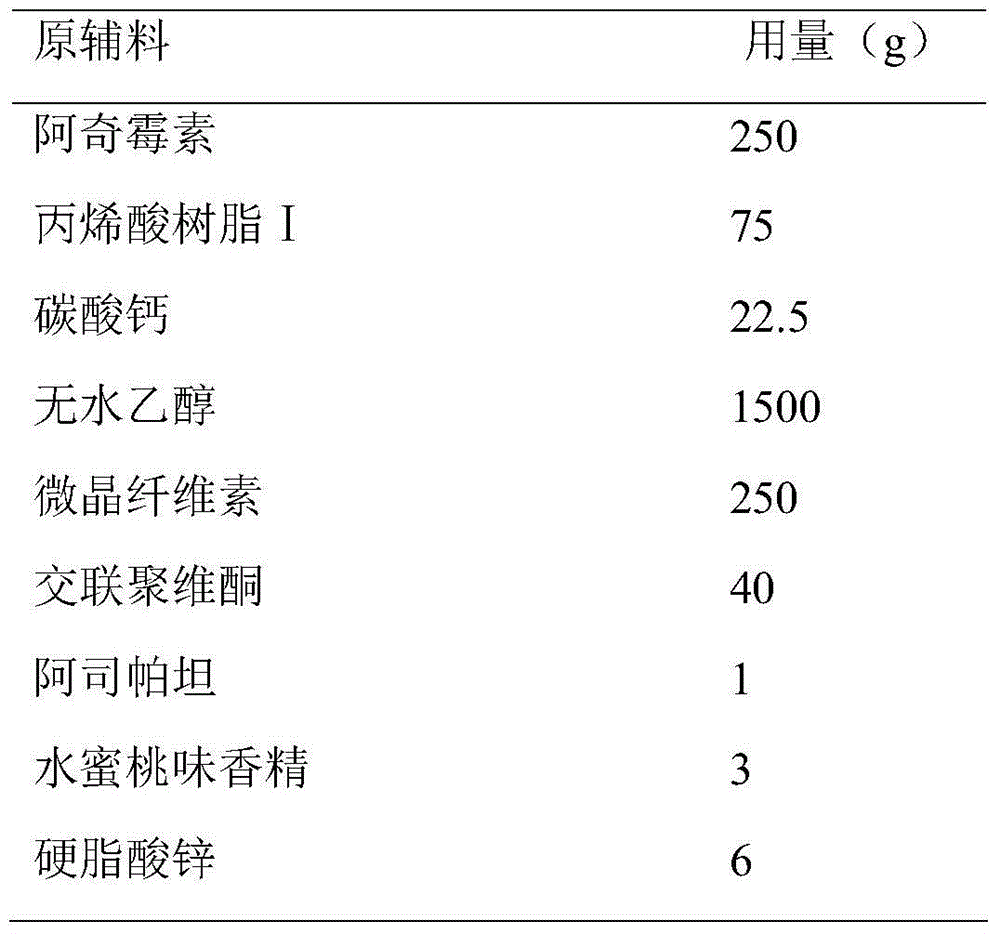
Azithromycin dispersible tablet
Owner:LUNAN BETTER PHARMA
Qingkailing dispersing tablet
ActiveCN1682836AHas the function of clearing heat, detoxifying, calming and calming the nervesAntipyreticAnalgesicsDiseaseAcute Pharyngitis
Owner:哈药集团三精千鹤制药有限公司
Anti-gout novel medical preparation
InactiveCN101152262ALower uric acid levelsPowder deliverySkeletal disorderFreeze-dryingOTERACIL POTASSIUM
The invention relates to the drug technical field and concretely provides a novel drug preparation for resisting the gout. The preparation includes the raw materials of two traditional Chinese medicines of a wormwood leaf and a plantain seed, which are mixed, dipped and boiled for obtaining a water-extract to be filtered, heated, condensed, centrifugally residue removed, freeze-dried, dissolved by normal saline, filtered by a filter membrane and finally made into the novel drug preparation for resisting the gout. The animal text shows that the novel drug preparation of the invention can very effectively reduce the oteracil potassium to induce the uric acid standard in the blood of a mouse with the hyperuricemia and greatly improve and effectively cure the mice of arthrocele and difficult action, and the novel drug is further used for resisting the human gout to take particular effect.
Owner:邱日辉 +1
'Xindakang' dispersion tablets and prepn. method
Owner:BEIJING INCREASEPHARM CORP LTD
Chinese medicinal formulation for treating spermophlebectasia
InactiveCN1965991APromote blood circulationImprove survival ratePowder deliveryUnknown materialsMyrrhRegimen
Owner:ZHEJIANG UNIV
Traditional Chinese medicine composition, preparation thereof and preparation method of preparation
InactiveCN105641619AEffusion is curativeDefinite curative effectPowder deliveryAnthropod material medical ingredientsAngelica Sinensis RootRhizome
Owner:葛小平
Cassia twig and poria cocos pills and preparation method thereof
InactiveCN106177026ALow costSmall side effectsPharmaceutical non-active ingredientsPill deliverySide effectRemove blood
Owner:上海典实医疗科技有限公司
Drug for treating rheumatoid arthritis
ActiveCN106880789AGood curative effectShorten the timeAnthropod material medical ingredientsAntipyreticAngelica Sinensis RootClinical manifestation
The invention discloses a drug for treating rheumatoid arthritis. The drug is prepared from radix aconiti kusnezoffi preparata, radix aconiti preparata, radix angelicae, asarum, lumbricus, rhizoma arisaematis, flos carthami, yam rhizome, semen coicis, radix angelica sinensis, placenta and ants according to a proper ratio. The medicines are ground into fine powder, and the fine powder is coated with raw honey to form pellets. Due to the fact that the toxicity of the formula is high, the drug needs to be stopped for two days to observe the pharmacodynamic function after being used for one week. The drug has a remarkable curative effect for rheumatoid arthritis, especially when clinical manifestations include symmetrical pain and swelling of multiple joints in the whole body, symmetrical stiffness or tightness of joints, even deformation to different degrees, alternate chills and fever, limb joint and muscular ache, unsmooth flexion and extension, or unstable pain, and even severe pain, swelling, hardness and deformation of joints. Treatment of 200 patients confirmed through blood and modern medical equipment proves that 166 patients are cured, 11 patients are improved, the effective rate is 88.5%, and the cure rate is 83%.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
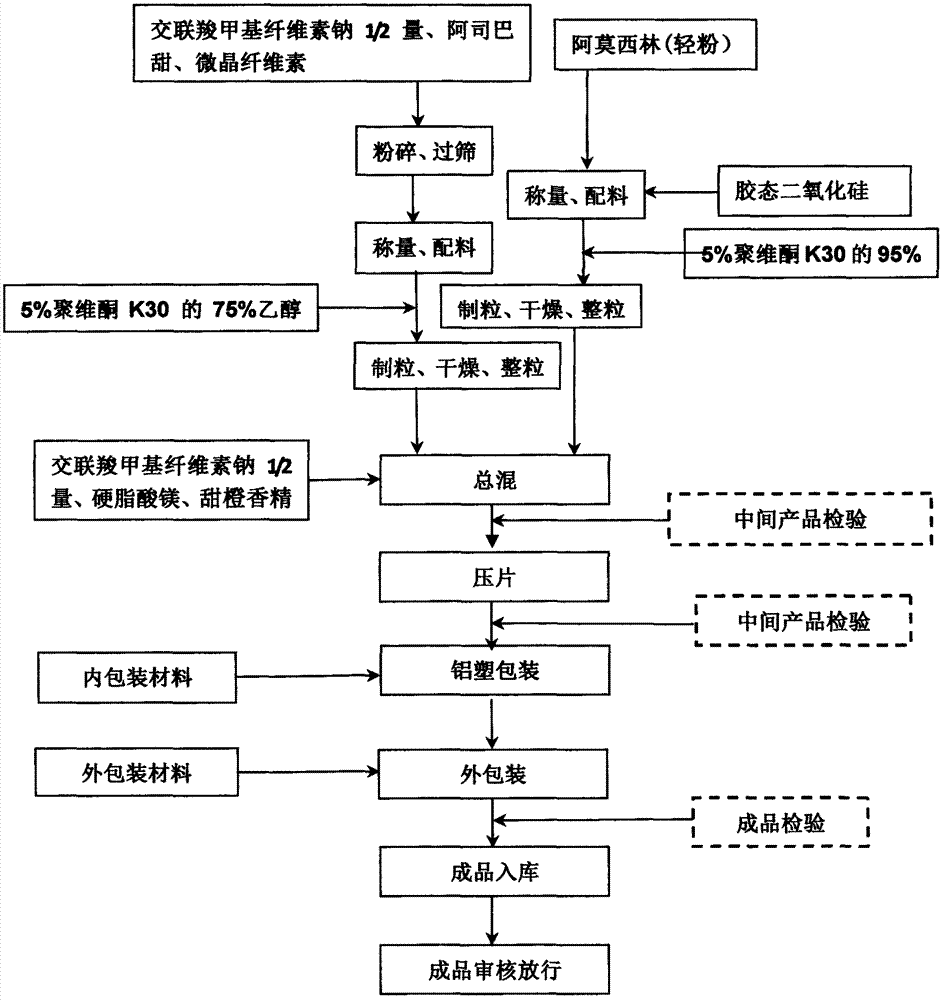
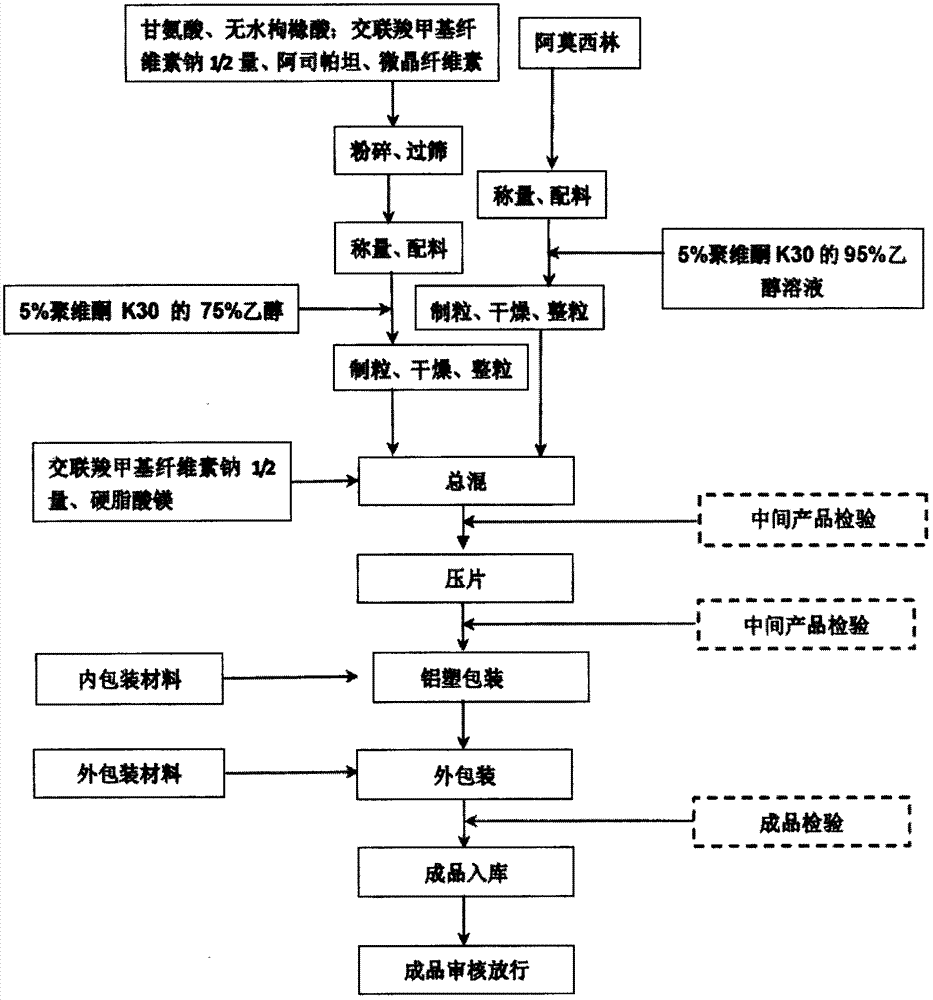
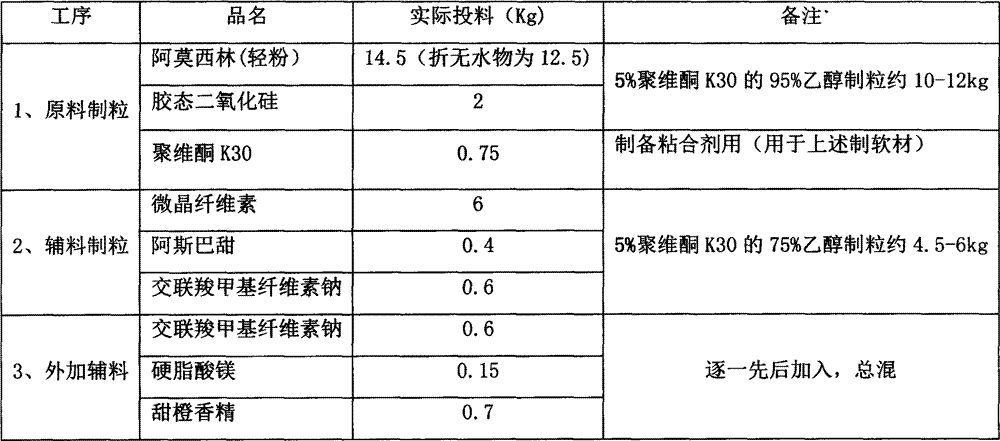
Amoxicillin orally disintegrating tablet and preparation method thereof
InactiveCN107115305AStable traitsDisintegration time as expectedAntibacterial agentsDigestive systemMedicineOrally disintegrating tablet
Owner:NINGBO SHUANGWEI PHARMA
Composition of hydrophilic polymer
Owner:钟术光
Glipizide orally disintegrating tablet and preparation method thereof
InactiveCN107412175AEvenly dispersedReduce usageMetabolism disorderSulfonylurea active ingredientsFiller ExcipientIrritation
The invention discloses a glipizide orally disintegrating tablet and a preparation method of the glipizide orally disintegrating tablet. The orally disintegrating tablet comprises glipizide and pharmaceutically acceptable auxiliary materials with effective doses, wherein the pharmaceutically acceptable auxiliary materials comprise a filling agent, a flavoring agent, a disintegrating agent, a lubricant and an adhesive; the filling agent is composed of mannitol and microcrystalline cellulose; the flavoring agent is mannitol; the disintegrating agent is a composition of crospovidone, low-substituted hydroxypropyl cellulose and sodium carboxymethyl starch, and the weight ratio of the crospovidone to the low-substituted hydroxypropyl cellulose to the sodium carboxymethyl starch is (5-20): (5-30): (2-10); the lubricant is magnesium stearate; the adhesive is purified water. The product can rapidly disintegrate in the oral cavity, and is good in taste, easy to swallow, and free of irritation to the oral mucosa. The glipizide orally disintegrating tablet prepared by adopting the preparation method disclosed by the invention is uniform, meanwhile, the production cost is low, and thus large-scale production can be realized.
Owner:CHONGQING CONQUER PHARML
Preparation method of domperidone tablets
ActiveCN109481410AIncrease productivityHigh tablet hardnessOrganic active ingredientsDigestive systemCelluloseMagnesium stearate
The invention provides a preparation method of domperidone tablets, comprising the following steps: (1) material preparation: performing site-clearing inspection, getting a raw material domperidone, and ingredients lactose G200, pregelatinized starch, low-substituted hydroxypropyl cellulose, povidone K30, lauryl sodium sulfate, silica and magnesium stearate; (2) pelleting: sieving and uniformly mixing raw material and ingredients except povidone K30, silica and magnesium stearate, then slowly adding a povidone K30 solution, uniformly stirring, and sieving to prepare wet particles, drying, sieving and collecting to obtain dry particles; (3) generally blending: respectively adding silica and magnesium stearate into the dry particles obtained in the step (2), to obtain generally mixed particles; and (4) tabletting, and the like. The method is high in production efficiency, and can enable the prepared domperidone tablets to be high in dissolving-out speed and relatively long in expirationdate while ensuring relatively high streptomycin hardness.
Owner:WEIAO PHARM (SICHUAN) CO LTD
Traditional Chinese medicine porous core-shell type composite particle powder as well as preparation method and application thereof
InactiveCN113081971AIncreased porous structureFlat surfacePowder deliveryDigestive systemFoaming agentSpray dried
The invention discloses traditional Chinese medicine porous core-shell type composite particle powder as well as a preparation method and application thereof. The preparation method comprises the following steps: extracting traditional Chinese medicines by adopting a solvent, then filtering and concentrating to obtain a traditional Chinese medicine extraction concentrated solution, mixing the traditional Chinese medicine extraction concentrated solution, a shell layer material and a pore-foaming agent, stirring and dissolving the mixture to be uniform, and performing spray drying on the obtained mixture to obtain the traditional Chinese medicine porous core-shell type composite particle powder. The traditional Chinese medicine porous core-shell type composite particle powder prepared by the process provided by the invention has a remarkable porous structure, is spherical or sphere-like, and has a smooth surface; besides, the co-spray drying process adopted by the invention can be used for directly preparing the porous core-shell type particle powder in the spray drying stage of the traditional Chinese medicine extraction concentrated solution, so that the method is beneficial to process amplification for actual production, and is suitable for continuous production; moreover, after the traditional Chinese medicine porous core-shell type composite particle powder provided by the invention and a pharmaceutically acceptable carrier are prepared into tablets through direct pressing, the obtained tablets disintegrate faster.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Gastric retentive pharmaceutical compositions for extended release of polypeptides
Owner:DEPOMED SYST INC
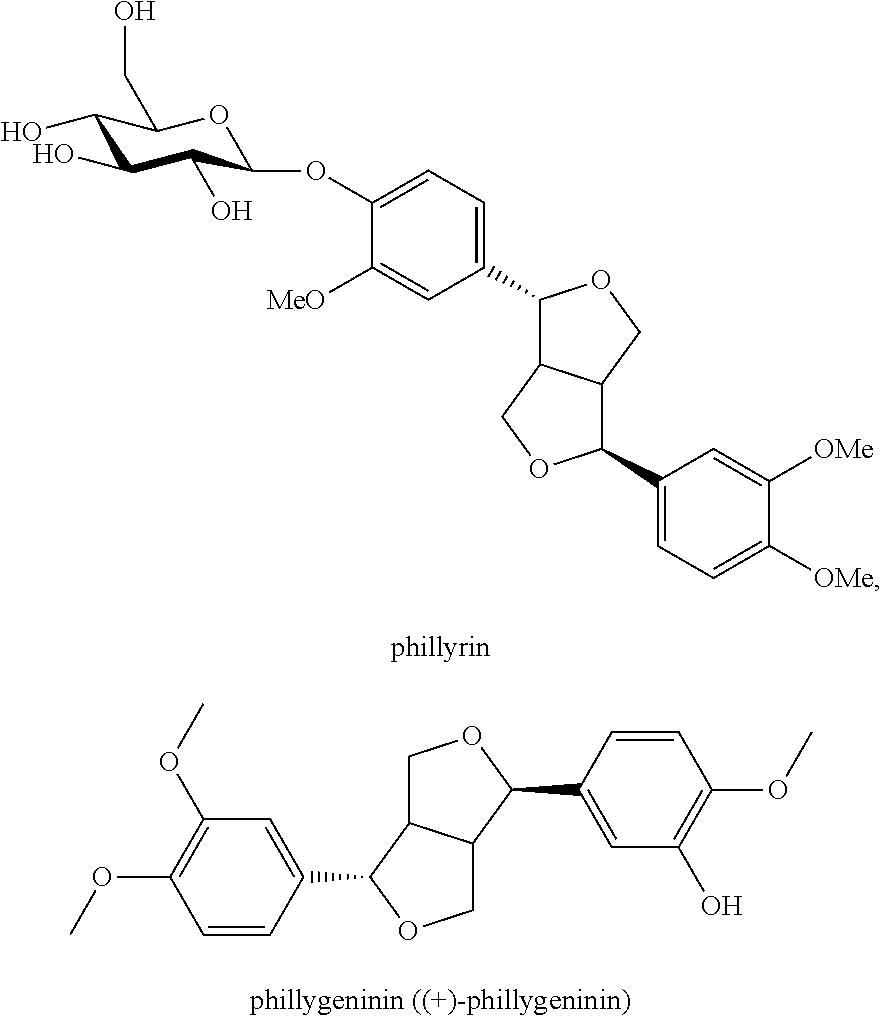
Application of a phillyrin/phillygeninin composition in preparing a medicine or health care product for alleviating or/and treating viral diseases, and medicine or health care product for treating viral diseases
ActiveUS20170232025A1Remarkable effectivenessSuitableAntipyreticAnalgesicsSide effectTreatment effect
Owner:FU LI
Traditional Chinese medicine for treating yin-deficiency fire-hyperactivity type neurasthenia and preparation method thereof
InactiveCN105031185AStrong targetingDefinite curative effectNervous disorderPill deliveryMongolian oakSide effect
Owner:孙志清
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap