Gastric retentive pharmaceutical compositions for extended release of polypeptides
a technology of gastric retentive and pharmaceutical compositions, which is applied in the directions of drug compositions, pharmaceutical delivery mechanisms, peptide/protein ingredients, etc., can solve the problems of insufficient digestion of meals, the oral delivery of therapeutic peptides or polypeptide compositions,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation of Prototype I Gastric Retentive Tablets Having Pancreatin Micropellets
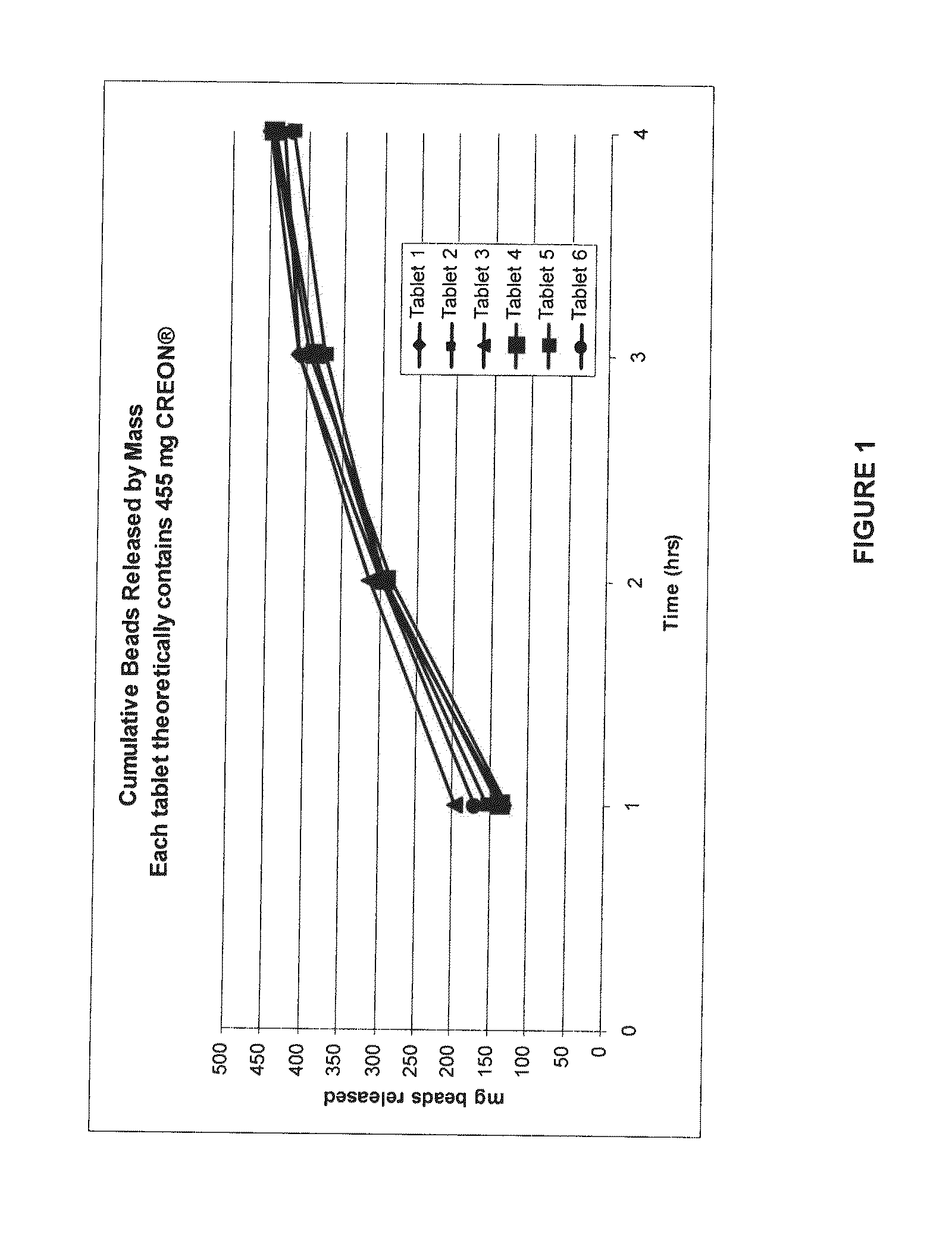
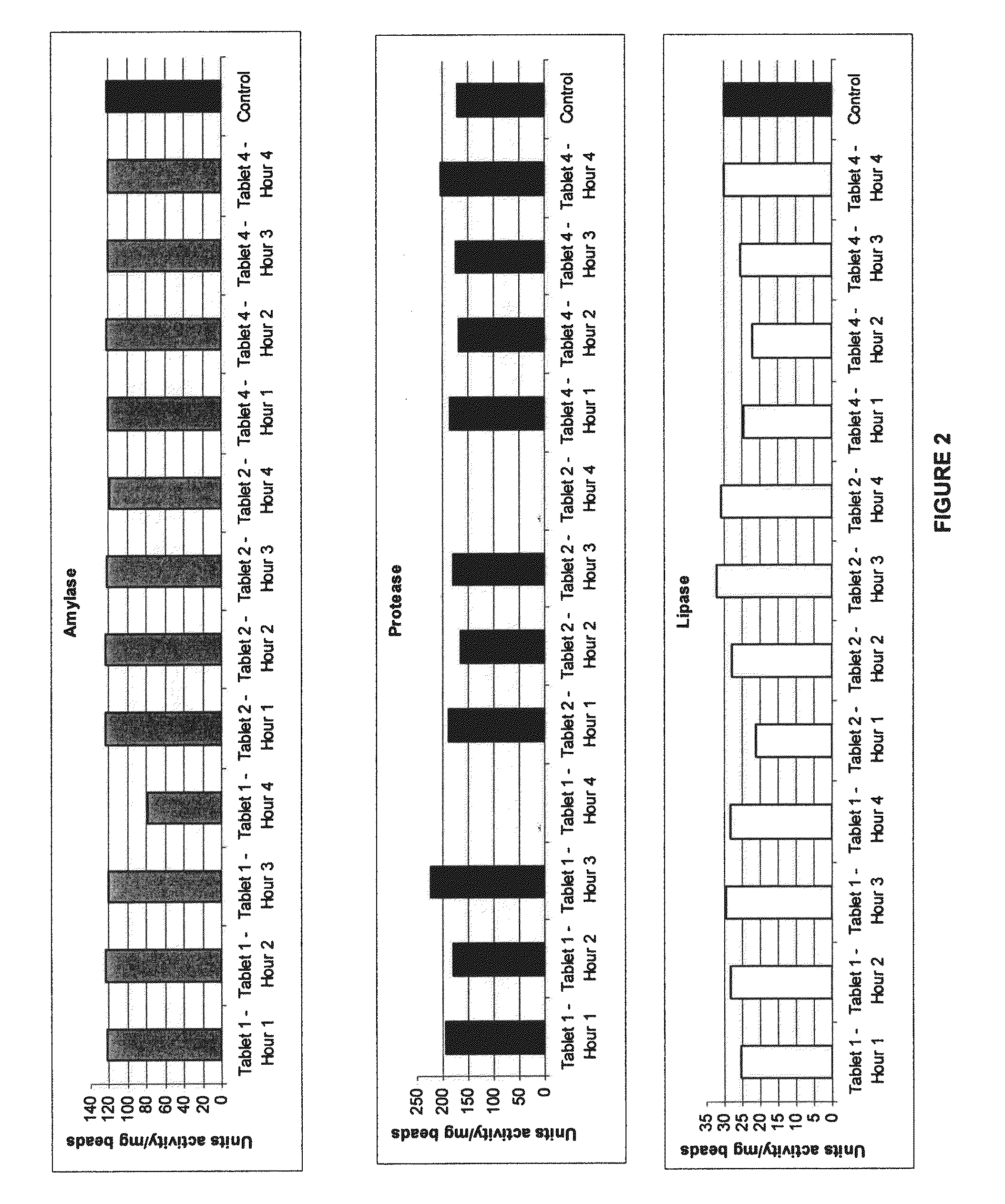
[0184]Gastric retentive tablets containing CREON 1224 pellets were manufactured and evaluated. This experiment was done to investigate the ability of the dosage form to deliver the pellets over 4 hours. Enzymatic activity provided by the released pellets was assayed following dissolution of the oral dosage form in acidic pH.
[0185]CREON 1224 pellets were compressed in a matrix formulation designed to swell upon hydration to a size which would allow gastric retention of the dosage form in a stomach in the fed mode. A blend of excipients, as provided in Table 1, was thoroughly mixed using a dry blend process. An amount of the resultant blend required to make a 1000 mg tablet was weighed out onto a weigh paper. The enteric coated pellet contents of a CREON 1224 capsule was emptied onto the weigh paper with the blend. Using a small spatula, the pellets and excipients were carefully mixed prior to being filled
example 2
Potential Patient Benefit of Optimized Mixing of Pancreatic Enzymes and Food
[0202]As a means of investigating whether patients would benefit from optimized mixing of pancreatic replacement therapy (i.e. pancrelipase) and food over the course of gastric emptying of a meal, a study is done to compare a gastric retentive oral dosage form, designed to deliver the enzymes throughout the meal, at various doses (as measured by Lipase units) with an existing product, for instance, CREON 1224, which delivers all the enzymes at the beginning of the meal.
[0203]Thirty-six Cystic Fibrosis (CF) patients already on existing pancreatic enzyme replacement therapy, aged 7-18 years old, are dosed at a specific dose within the label recommendation of existing therapy, in this case, CREON 1224 (see prescribing information). The patients are randomized into 4 cohorts and administered one of the four therapeutic regimen listed below, and then crossed over such that each patient will receive each therapeutic
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap