Amoxicillin orally disintegrating tablet and preparation method thereof
A technology of orally disintegrating tablets and amoxicillin, which is applied in the field of amoxicillin orally disintegrating tablets and its preparation, can solve problems such as unsatisfactory effects and ineffective treatment effects, and achieve stable properties, rapid results, and convenient oral administration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
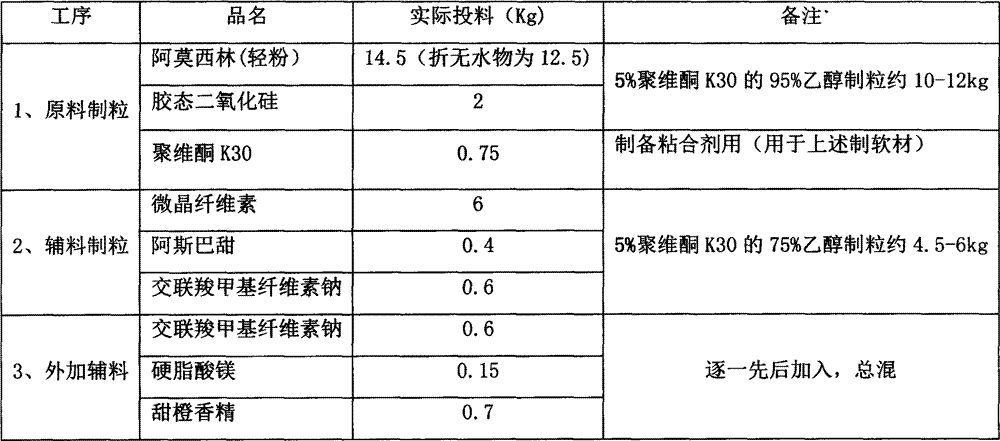
[0025] Concrete each component consumption ratio is as shown in table 1 below:
[0026] Table 1
[0027]
[0028] Concrete preparation steps:
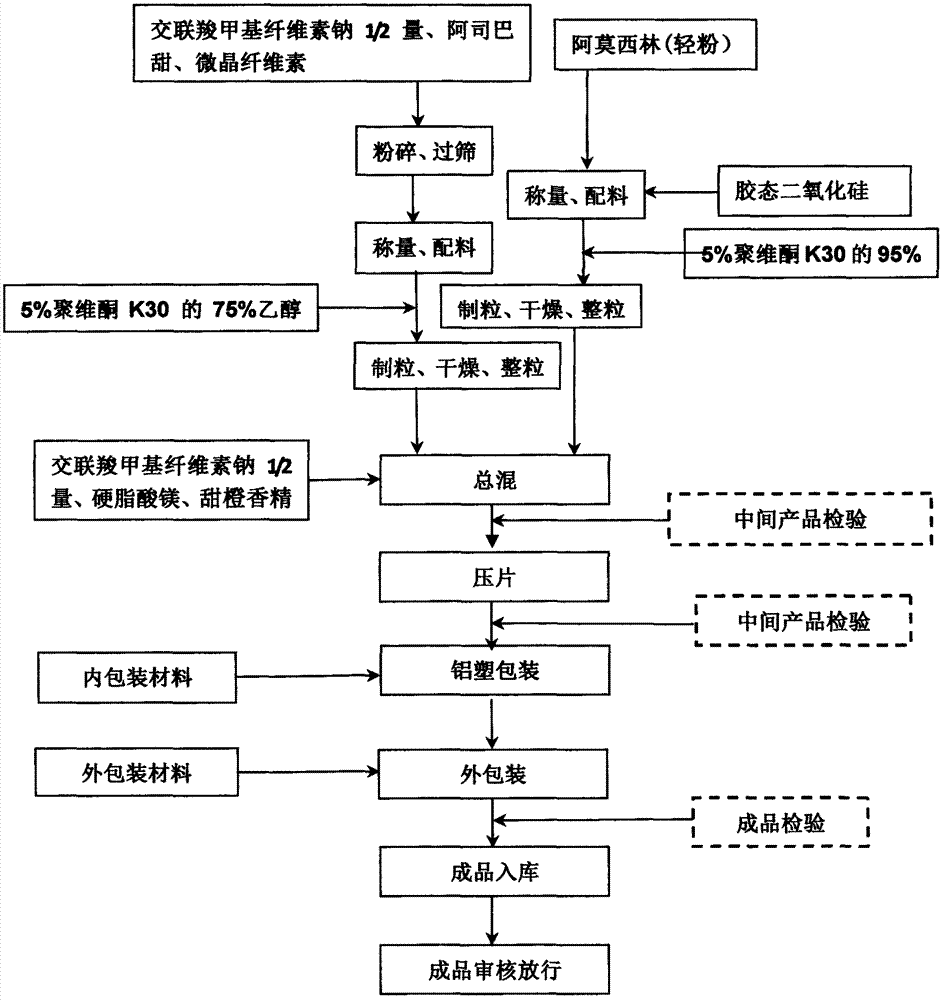
[0029] 1. Main drug granules: Amoxicillin (light powder) and silicon dioxide are placed in a wet granulator and mixed evenly, then an appropriate amount of 95% ethanol (volume percentage) of 5% K30 is added, the soft material is passed through 18 mesh, and dried at 40°C After 3-4 hours, granulate with 18 mesh sieve.
[0030] 2. Auxiliary material granules: except those added, use 5% K30 (75% ethanol) solution in an appropriate amount for the remaining adjuvant (croscarmellose sodium 1 / 2). Pass the soft material through 18-mesh sieve, dry at 60°C for 3-4 hours, and granulate with 18-mesh sieve.
[0031] 3. Total blending: main drug granules, excipient granules and external excipients.
[0032] 4. Tablet compression: Tablet compression is performed according to the particle content, and the pressure is controlled at about 1.5-3KG t
Embodiment 2
[0051] Table 7 Small scale scale-up of 100,000 prescriptions
[0052]
[0053] Concrete preparation steps are:
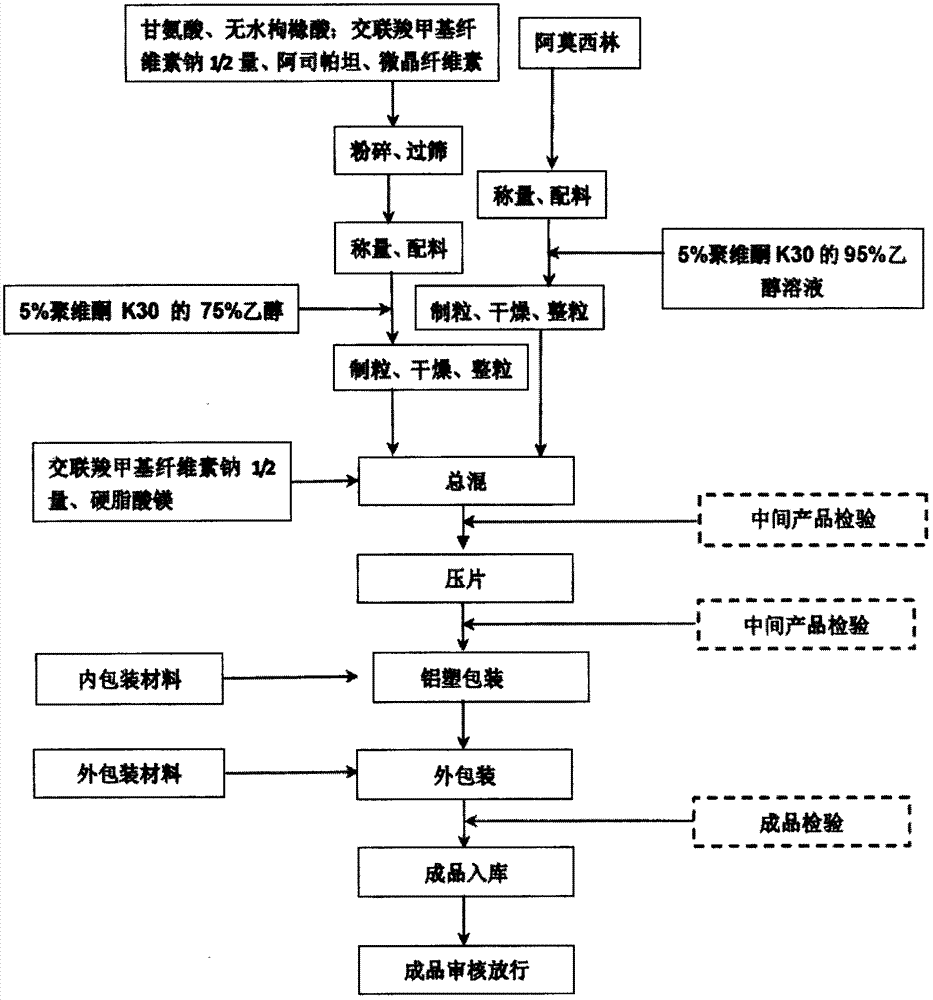
[0054] 1. Glycine and anhydrous citric acid were crushed to 80 meshes respectively. The croscarmellose sodium, microcrystalline cellulose and aspartame were respectively passed through an 80-mesh sieve. The crushed and sieved raw and auxiliary materials are stored in a transfer barrel with a clean plastic bag and covered. Attach the material label and transport it to the weighing and batching room. The crushed and sieved raw materials should be used within 10 days.
[0055] 2. Granulation and mixing
[0056] a. Preparation of adhesive
[0057] Adhesive I: 0.4kg of povidone K30 was placed in a stainless steel barrel, and 8L of 95% ethanol was added, and stirred slowly until it was completely dissolved, to prepare a 5% povidone K30 ethanol solution as the adhesive I.
[0058] Adhesive II: 0.35kg of povidone K30 was placed in a stainless steel barrel, and 7L of 7
Embodiment 3
[0103] Table 15 Screening prescriptions (designed according to each prescription)
[0104]
[0105] The preparation process is the same as in Example 2.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap