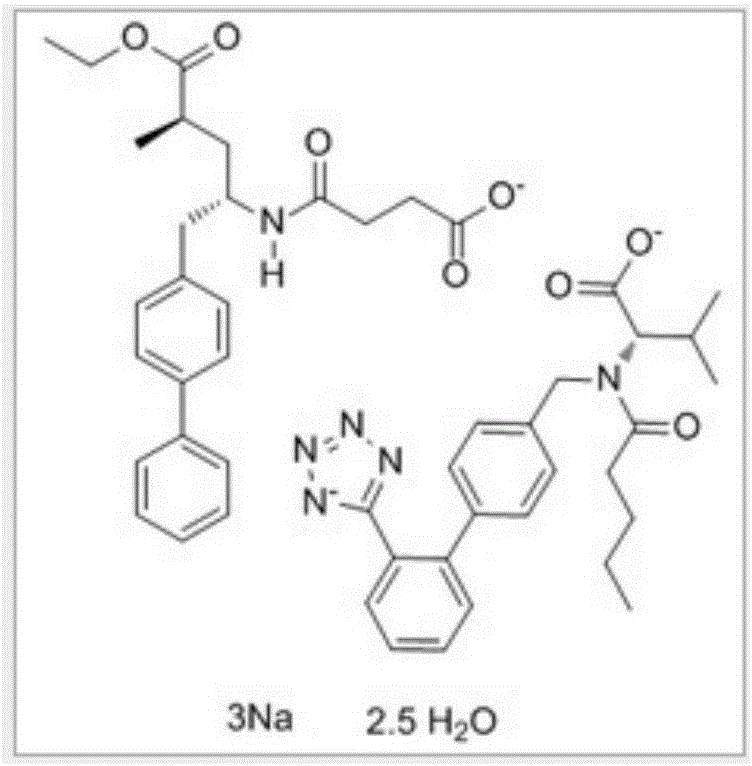
Preparation method of LCZ696 sustained release matrix tablet for treatment of heart failure
A technology for slow-release skeletons and skeleton materials, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. The effect of stable drug concentration and slow drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
4
[0031] Preparation process: Raw and auxiliary materials are passed through 80-mesh sieve respectively, and the LCZ696 raw material is weighed according to the prescription and mixed with hypromellose K15M, and then mixed evenly with mannitol, micronized silica gel, talcum powder, and magnesium stearate, and the whole powder is directly pressed piece.
[0032] For the release test, the release rate of the sample was measured according to the first method of the release rate of the four general rules of the Chinese Pharmacopoeia 2015 edition. With 900ml of buffer salt of pH6.8 as solvent, 100 revolutions of rotating speed, sampling in 1, 4 and 8 hours, measure the release rate of sample, the result is as follows:
[0033] time
Embodiment 2
2
[0036] Preparation process: Raw and auxiliary materials are passed through 80-mesh sieve respectively, and the LCZ696 raw material is weighed according to the prescription and mixed with hypromellose K15M, and then mixed evenly with mannitol, micronized silica gel, talcum powder, and magnesium stearate, and the whole powder is directly pressed piece.
[0037] For the release test, the release rate of the sample was measured according to the first method of the release rate of the four general rules of the Chinese Pharmacopoeia 2015 edition. With 900ml of buffer salt of pH6.8 as solvent, 100 revolutions of rotating speed, sampling in 1, 4 and 8 hours, measure the release rate of sample, the result is as follows:
[0038] time
Embodiment 3
4
[0041] Preparation process: The raw and auxiliary materials are passed through 80-mesh sieve respectively, and the raw materials of LCZ696 and hypromellose K15M are weighed according to the prescription, mixed evenly, and then mixed evenly with mannitol, micronized silica gel, talcum powder, and magnesium stearate, and the whole powder is directly Tablet.
[0042] For the release test, the release rate of the sample was measured according to the first method of the release rate of the four general rules of the Chinese Pharmacopoeia 2015 edition. With 900ml of buffer salt of pH6.8 as solvent, 100 revolutions of rotating speed, sampling in 1, 4 and 8 hours, measure the release rate of sample, the result is as follows:
[0043] time
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap