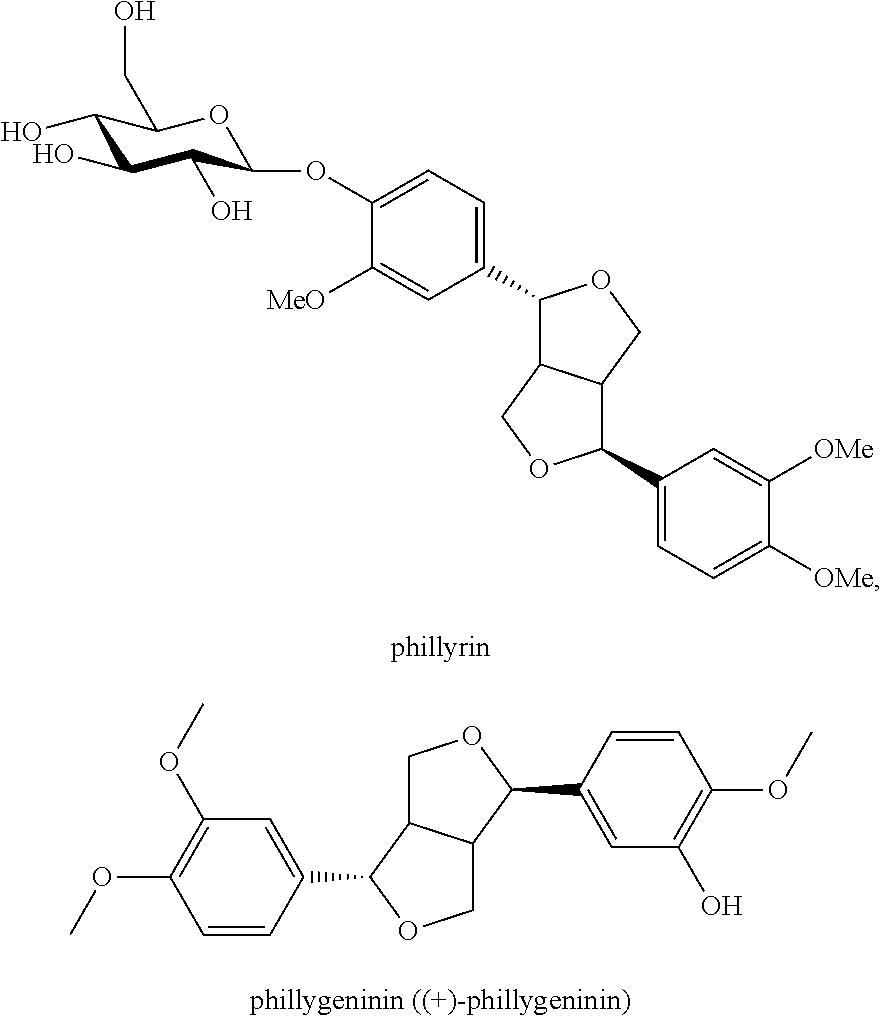
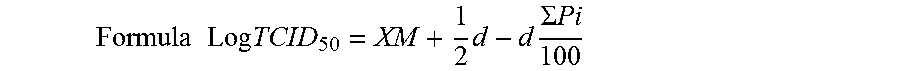Application of a phillyrin/phillygeninin composition in preparing a medicine or health care product for alleviating or/and treating viral diseases, and medicine or health care product for treating viral diseases
a technology of phillyrin and composition, applied in the field of pharmaceutical chemistry, can solve the problems of no natural drugs capable of effectively treating viral influenza and pneumonia in the world, serious harm to human health, and inability to meet the needs of industrial production, and achieves remarkable resistance and antiviral effect. better, suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-4
Preparation of the Phillyrin / Phillygeninin Composition
[0048]Two monomer component powders, i.e., Phillyrin and phillygeninin, were separately weighted to mix according to the weight ratio shown in Table 1, to prepare a phillyrin / phillygeninin composition; the phillyrin monomer was manufactured by Dalian Fusheng Natural Drug Development Co., Ltd.; the purity thereof was determined to be 99.5%, determined by HPLC equipped with both UV detector and evaporative light-scattering detector (ELSD) using area normalization method, and the content thereof was calibrated and confirmed to be 99.5% with phillyrin standard available from China Pharmaceutical and Biological Products for content determination; phillygeninin was manufactured by Dalian Fusheng Natural Drug Development Co., Ltd., the purity thereof was determined to be 99.1%, determined by HPLC equipped with both UV detector and evaporative light-scattering detector (ELSD) using area normalization method
TABLE 1Raw material ratio tabl
examples 5-24
Preparation of the Phillyrin / Phillygeninin Composition
[0049]The phillyrin / phillygeninin composition prepared in Example 1-4 was taken to be prepared into a composition comprising cydodextrin according to the weight ratio shown in Table 2 by using the following method: (1) directly adding to a cydodextrin solution, or (2) directly adding to a cydodextrin solution and well stirring for 1-24 h, (3) directly adding to a cydodextrin solution and heating for 10-120 min, (4) directly adding to a cydodextrin solution and performing ultrasonic treatment for 120 min, (5) directly grinding together with cydodextrin powder for 10-120 min, (6) mixing the phillyrin / phillygeninin composition well with the cydodextrin powder and sieving the mixture; (7) directly adding to a cydodextrin derivative solution, or (8) directly adding to a cydodextrin derivative solution and well stirring for 1-24 h, (9) directly adding to a cydodextrin derivative solution and heating for 10-120 min, (10) directly add
example 25 preparation
of the Phillyrin and Phillygeninin Composition
[0051]10 kg of 95% (n / m) ethanol was added to 1 kg of dried leaves of Forsythia suspensa, the mixture was reflux-extracted under heating twice for 2 h each time, the extracted liquid was filtered, the filtrate was concentrated under vacuum to ½ of the original volume, and was allowed to stand for precipitating at 25° C. for 1 h to separate out precipitates; the precipitates were dissolved with methanol for recrystallization, and precipitates were separated out; the above process was repeated for recrystallization with methanol to obtain an amorphous powder of a phillyrin / phillygeninin composition, with the contents of phillyrin and phillygeninin being 98% and 2% respectively, as determined by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight ratio | aaaaa | aaaaa |
| Ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap