Hydrogen-bond self-assembly super-molecular blue-fluorescence polymer and symmetric method thereof
A technology of blue fluorescence and synthesis method, applied in the field of pyrazoline compounds, can solve problems such as poor performance of electroluminescent devices, and achieve the effects of improving connection strength, improving fluorescence quantum yield, and increasing bonding strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
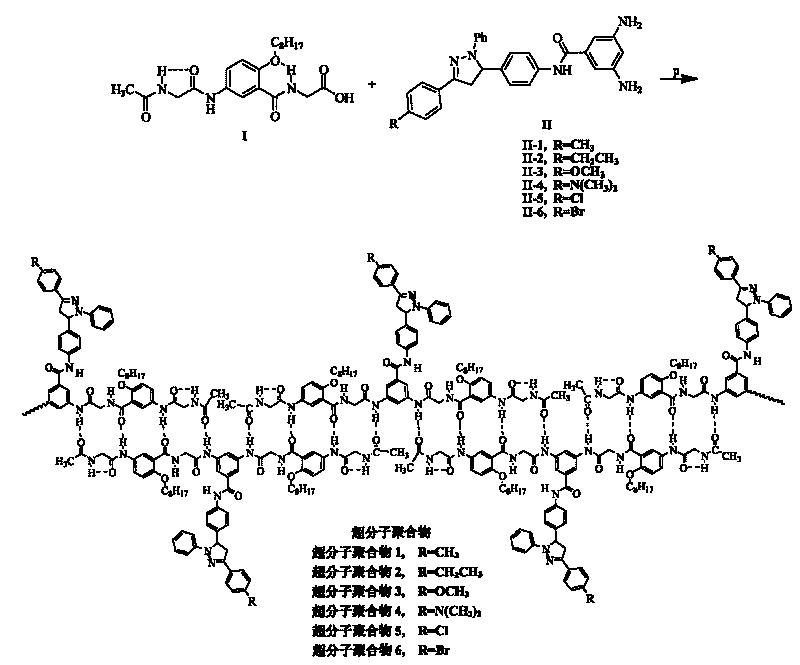
[0088] Supramolecular polymer 1 and its synthesis:
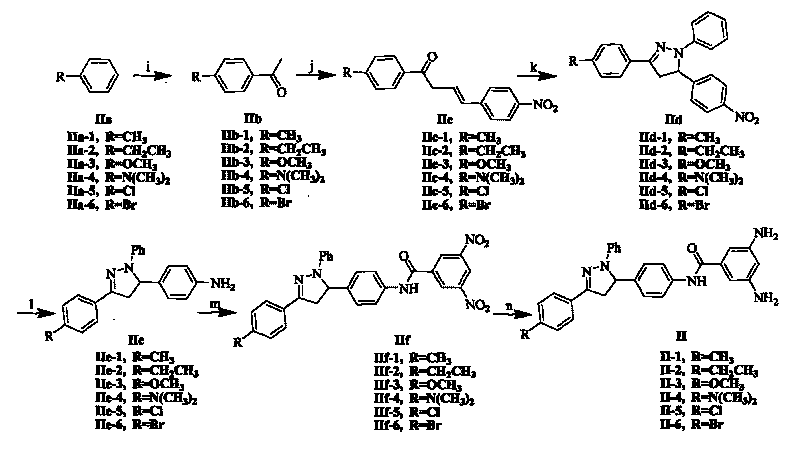
[0089] The synthetic process step of supramolecular polymer 1 comprises following three steps: (1) synthetic N-carboxymethyl {2-octyloxy group-5-[N-(2-acetamidoacetyl) amino] benzamide }(fragment I); (2) synthesis of 3,5-diamino-N-{4-[1-phenyl-3-(4-methylphenyl)-4,5-dihydro-1H-pyrazole -5-yl] phenyl} benzamide (fragment II-1); (3) link fragment I and fragment II-1 into monomer molecule 1, and self-assemble into supramolecular polymer 1.
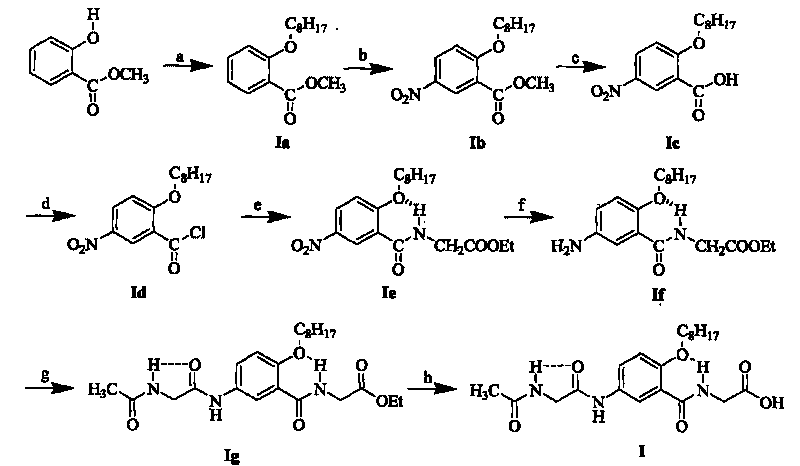
[0090] 1. Synthesis of Fragment I
[0091] Fragment I was successfully prepared from methyl salicylate as raw material through eight steps of reaction and seven intermediates including Ia, Ib, Ic, Id, Ie, If, and Ig. Among them, the process steps (1) to (6) of the first six steps of reaction, that is, the synthesis process steps of intermediates Ia, Ib, Ic, Id, Ie, If, can be found in Chinese invention patent ZL200410081544.1, patent application date: 2004 December 21.
[0092] (7) Synth...
Embodiment 2
[0113] Supramolecular polymer 2 and its synthesis:
[0114] The synthesis process step of supramolecular polymer 2 comprises following three steps: (1) synthetic N-carboxymethyl {2-octyloxy group-5-[N-(2-acetamidoacetyl) amino] benzamide }(fragment I); (2) synthesis of 3,5-diamino-N-{4-[1-phenyl-3-(4-ethylphenyl)-4,5-dihydro-1H-pyrazole -5-yl] phenyl} benzamide (fragment II-2); (3) link fragment I and fragment II-2 into monomer molecule 2, and self-assemble into supramolecular polymer 2.
[0115] The synthesis process steps of Fragment I are the same as in Example 1; the synthesis process steps of Fragment II-2 are similar to the synthesis process steps of Example 1 Fragment II-1, only the raw material is replaced by ethylbenzene (IIa-1) by toluene (IIa-1) 2); the synthesis process steps of linking fragment I and fragment II-2 into monomer molecule 2 and self-assembling into supramolecular polymer 2 are the same as in Example 1, only replacing II-1 with II-2. The obtained sup...
Embodiment 3
[0117] Supramolecular polymer 3 and its synthesis:
[0118] The synthetic process step of supramolecular polymer 3 comprises following three steps: (1) synthetic N-carboxymethyl {2-octyloxy group-5-[N-(2-acetamidoacetyl) amino] benzamide } (fragment I); (2) synthesis of 3,5-diamino-N-{4-[1-phenyl-3-(4-methoxyphenyl)-4,5-dihydro-1H-pyridine Azol-5-yl]phenyl}benzamide (fragment II-3); (3) link fragment I and fragment II-3 into monomer molecule 3, and self-assemble into supramolecular polymer 3.
[0119] The synthesis process steps of Fragment I are the same as in Example 1; the synthesis process steps of Fragment II-3 are similar to the synthesis process steps of Example 1 Fragment II-1, only the raw material is replaced by anisole (IIa-1) by toluene (IIa-1) -3); The synthesis process steps of linking fragment I and fragment II-3 into monomer molecule 3 and self-assembling into supramolecular polymer 3 are the same as in Example 1, only replacing II-1 with II-3. The obtained su...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap