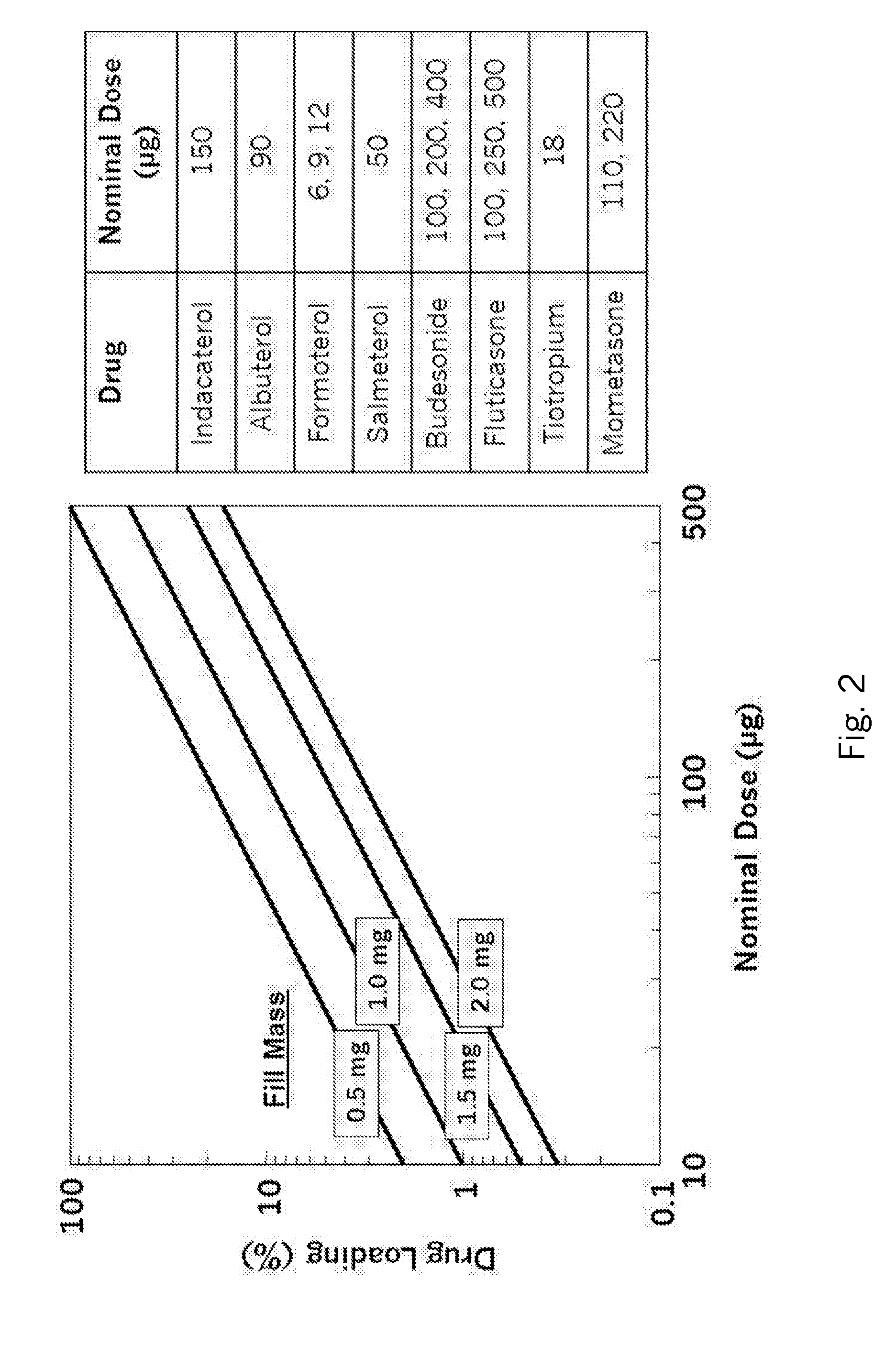
Deamorphization of spray-dried formulations via spray-blending
a technology of spray-dried formulations and spray-blending, which is applied in the direction of drug compositions, biocide, anti-inflammatory agents, etc., can solve the problems of micronized drug particle coarsening, thermodynamic instability of amorphous domains, and undesirable presence of crystalline micronized drugs for inhalation, so as to limit the content of amorphous drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Spray-Blended Dry Powder Formulations of Indacaterol Maleate and Indacaterol Maleate+Mometasone Furoate
[0214]In this Example, dry powder formulations of the invention containing indacaterol maleate were prepared by a spray-blending process. This includes a formulation comprising a fixed dose combination of indacaterol maleate and mometasone furoate.
[0215]Five spray-blended formulations (see Tables 3 and 4) were prepared and spray-dried on a Niro PS-1 scale spray-drier, equipped with a multi-headed HYDRA™ atomizer. The HYDRA™ atomizer (FIG. 3) contains five twin fluid nozzles (see FIG. 3A), each of which is controlled by an independent liquid feed line as shown schematically in FIG. 3B. A common gas line was used to supply atomization gas (compressed air) to all nozzles. The nozzles are oriented to minimize interaction of the spray plumes during drying. For the five lots detailed below, only three of the five spray nozzles were utilized (Feedlines A, B, C). The compositio
example 2
Preparation of Spray-Blended Dry Powder Formulations of Indacaterol Maleate and Indacaterol Maleate+Mometasone Furoate from an Emulsion-Based Feedstock
[0222]In this Example, more detail is provided on the preparation of the feedstocks used in Example 1. The dry powder formulations of the invention containing indacaterol maleate were prepared and dry powder formulations of the invention containing indacaterol maleate and mometasone furoate were prepared from an emulsion-based feedstock that was prepared in accordance with the method described in United States patent specification U.S. Pat. No. 6,565,885. In this process, crystalline micronized indacaterol maleate is dispersed in the continuous phase of an oil-in-water emulsion. The process resulted in crystalline indacaterol particles coated with a porous layer of hydrophobic excipient. The morphology of the particles was confirmed by scanning electron microscopy (data not shown).
[0223]Accordingly, distearoylphosphatidylcholine (DSPC) a
example 3
Measurement of the Physical Properties of Spray-Blended Dry Powder Formulations of Indacaterol Maleate and Indacaterol Maleate+Mometasone Furoate
[0230]In this Example certain physicochemical properties of the spray-blended dry powder formulations in Example 1 were measured, namely primary particle size, tapped density and water content.
[0231]FIGS. 7A-7E are photomicrographs of spray-blended powders of embodiments of the present invention, the powders comprising indacaterol. The powders were formulated according to Table 3, and FIGS. 7A-E correspond in order to the Lot I through Lot V. The powders exhibit the hollow, porous morphology characteristic of the emulsion-based spray drying process. There is no evidence of different types of particles in the spray-blended formulations of FIGS. 7A-E.
[0232]Table 6 presents the physical properties measured for those formulations.
TABLE 6Physical properties of spray-blended dry powder formulations ofindacaterol maleate and indacaterol maleate + mom
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap