Pharmaceutical compositions for poorly soluble drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0069] To produce the solid dispersion, a solution was prepared by dispersing HP-50 (420 g) in methylene chloride (8400 g) and then adding itraconazole (280 g) and stirring to form a pale brown solution. This solution was then spray dried to form a powder.
[0070] A portion (292 g) of this spray dried powder was then blended with sodium starch glycolate (93.6 g) and colloidal silicon dioxide (Aerosil 200)(5.6 g) in a Collette mixer at high speed for 5 minutes. Magnesium stearate (8.8 g) was added to the blend from the Collette mixer and the mixture tumble blended until uniform.
[0071] This powder blend was then filled into size 0 gelatin capsules by hand. Each capsule was filled with 345 to 359 mg of powder, containing nominally 98 to 102 mg of itraconazole.
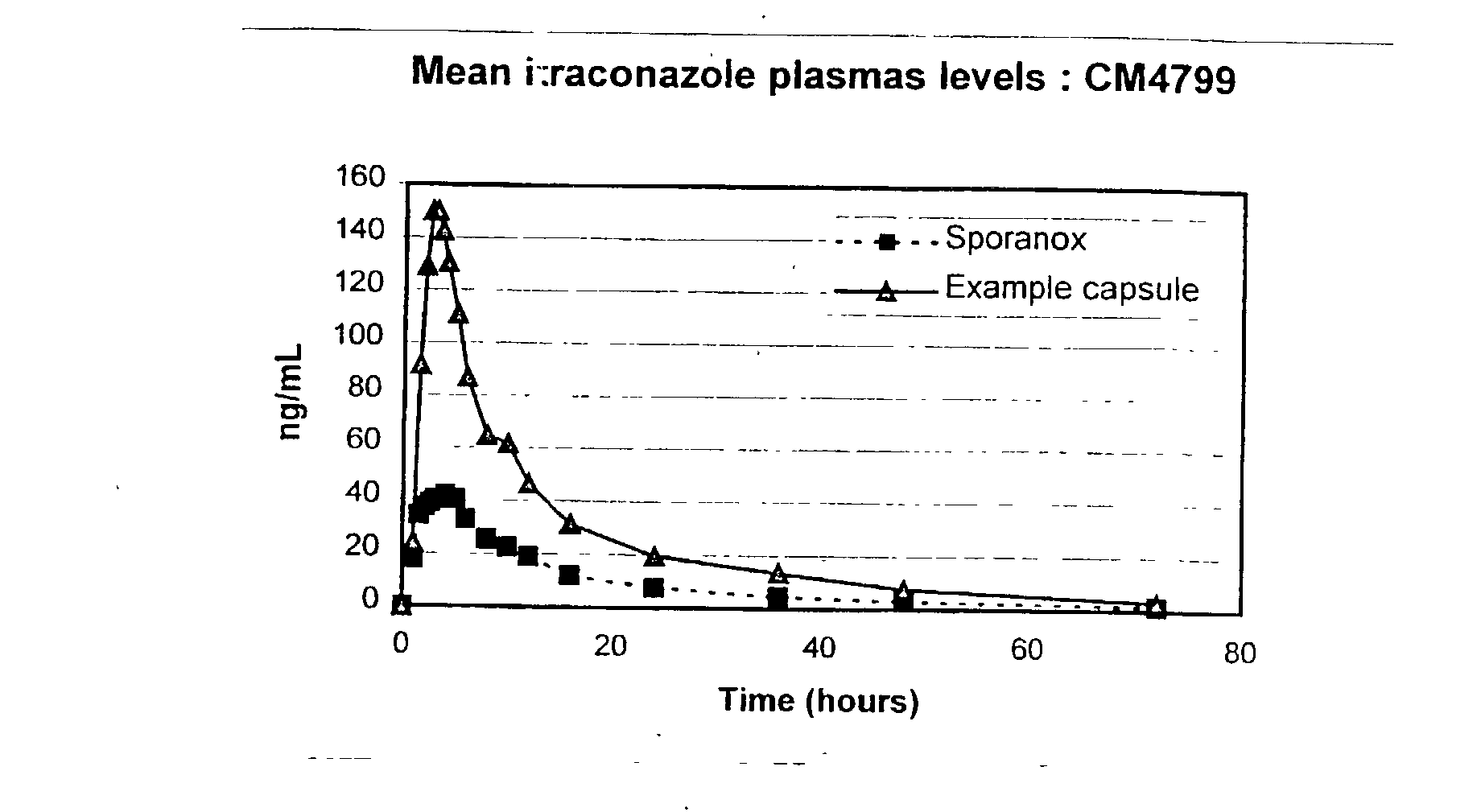
[0072] These test capsules were utilised in a pharmacokinetic study. 8 male volunteers were dosed with one 100 mg capsule after an overnight (10 hour) fast. The capsules were dosed with 240 ml water. At appropriate time intervals blood
example 3
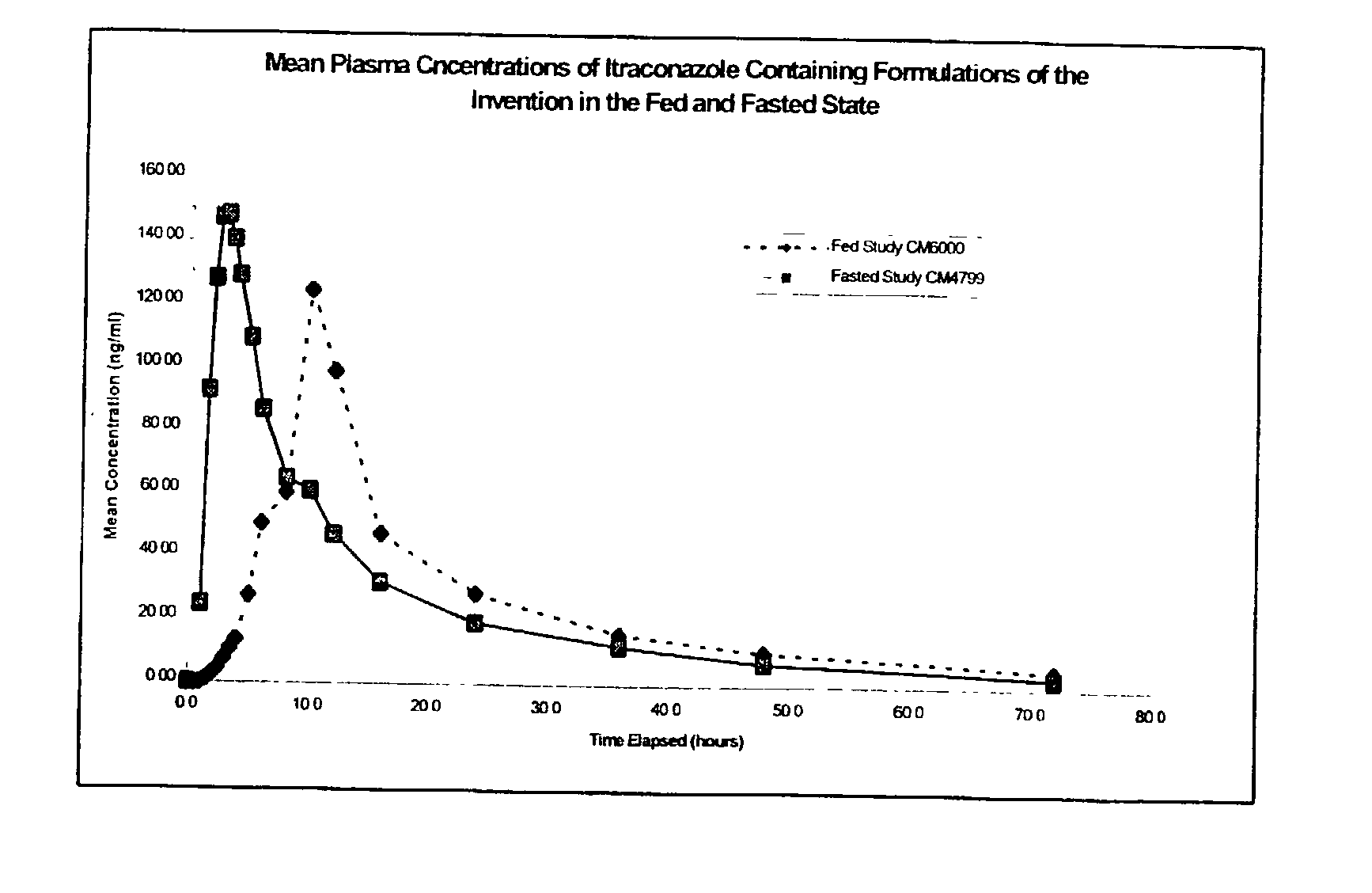
3 Example 3 Capsule Example 2 Capsule Parameter (Fed) (Fasted) C.sub.max (ng / ml) 148.20 182.6 T.sub.max (h) 10.25 2.94 AUC (ng .multidot. h / ml) 1806 1776 AUC.sub.inf (ng .multidot. h / ml) 1997 1875
[0083] It can be seen from these results that the example formulation produces plasma profiles considered bioequivalent in terms of AUC under fasting and fed conditions, due to the AUC under fed conditions being about 102% of the AUC under fasted conditions, which is well within the range of 80 to 120%. This is an indication that the total amount of drug absorbed over time is essentially equivalent under fed and fasted conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap