Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43results about "Disease diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Galectin-3 Immunoassay
InactiveUS20100143954A1Easy to manageImmunoglobulins against animals/humansDisease diagnosisEpitopeGalectin-3
The present invention relates to methods and compositions for specifically and quantitatively detecting galectin-3 in a sample. Embodiments of the invention include a detection assay in which a capture binding moiety and a labeled binding moiety specifically recognize non-overlapping epitopes on the N-terminus of galectin-3. Further embodiments are directed to a method for establishing ranges of galectin-3 concentrations indicative of the presence and severity of heart failure in a subject and a method for predicting the clinical outcome of a subject based upon galectin-3 concentration.
Owner:BG MEDICINE INC
Predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
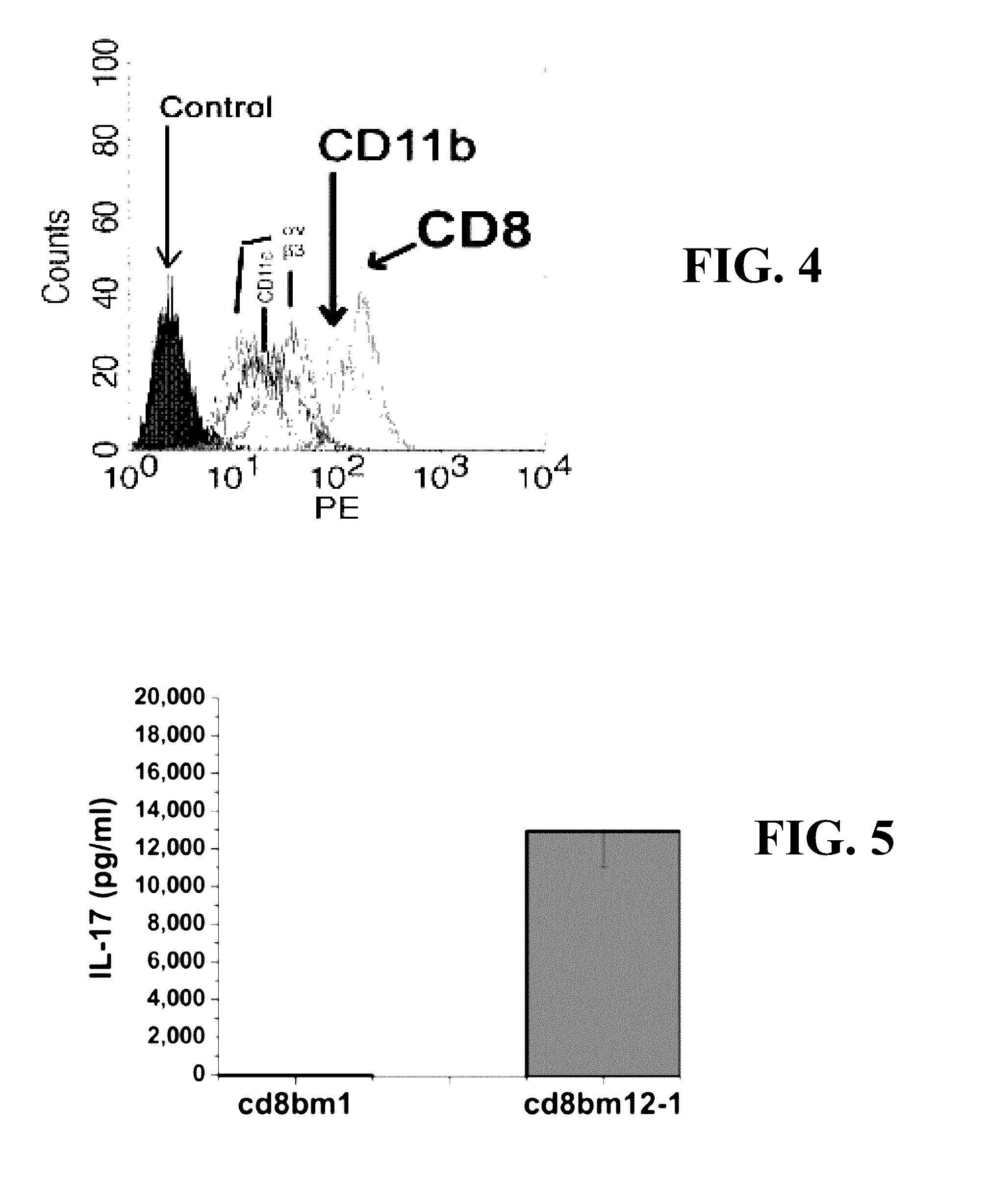
A method for treating a subject afflicted with an autoimmune disease with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of administering a therapeutic amount of the pharmaceutical composition to the subject, determining whether the subject is a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder by measuring the value of a biomarker selected from the group consisting of IL-10 concentration, IL-17 concentration, IL-18 concentration, TNF-α concentration, BDNF concentration, caspase-1 concentration, IL-10 / IL-18 ratio and IL-10 / IL-17 ratio in the blood of the subject, and comparing the measured value to a reference value for the biomarker to identify the subject as a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder, and continuing the administration if the subject is identified as a glatiramer acetate responder, or modifying treatment of the subject if the subject is identified as a glatiramer acetate hypo- / non-responder.
Owner:TEVA PHARMA IND LTD
Methods for Assessing the Risk for Development of Cardiovascular Disease
InactiveUS20080261250A1Low and prevent stressImprove the level ofMicrobiological testing/measurementDisease diagnosisDiagnostic testHematological test
The present invention relates to diagnostic tests, methods and kits that are useful to assess a subject's risk of developing a pathologic condition related in part to the presence of HDL oxidation product. Measuring the quantity of one or more HDL oxidation products present in the blood is useful in evaluating risk for developing or evaluating the severity of a disease or evaluating response to treatment for such a disease as, for instance, cardiovascular disease.
Owner:UNIV OF WASHINGTON
Prostate Cancer Biomarkers
ActiveUS20170010269A1Easy to manageConvenient treatmentDisease diagnosisEnzymesProstate cancerOncology
A method is provided for characterising and / or prognosing prostate cancer in a subject comprising measuring the level of at least one protein from a panel or at least one peptide thereof in a sample from the subject. The method may be used to determine the grade and stage of the prostate cancer. Also disclosed is a method for selecting a treatment for prostate cancer, together with corresponding methods of treatment. Systems and computing devices for performing the methods are also provided.
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
Method for Treating Ocular Cancer
Owner:FLANDERS INTERUNIV INST FOR BIOTECH VIB +3
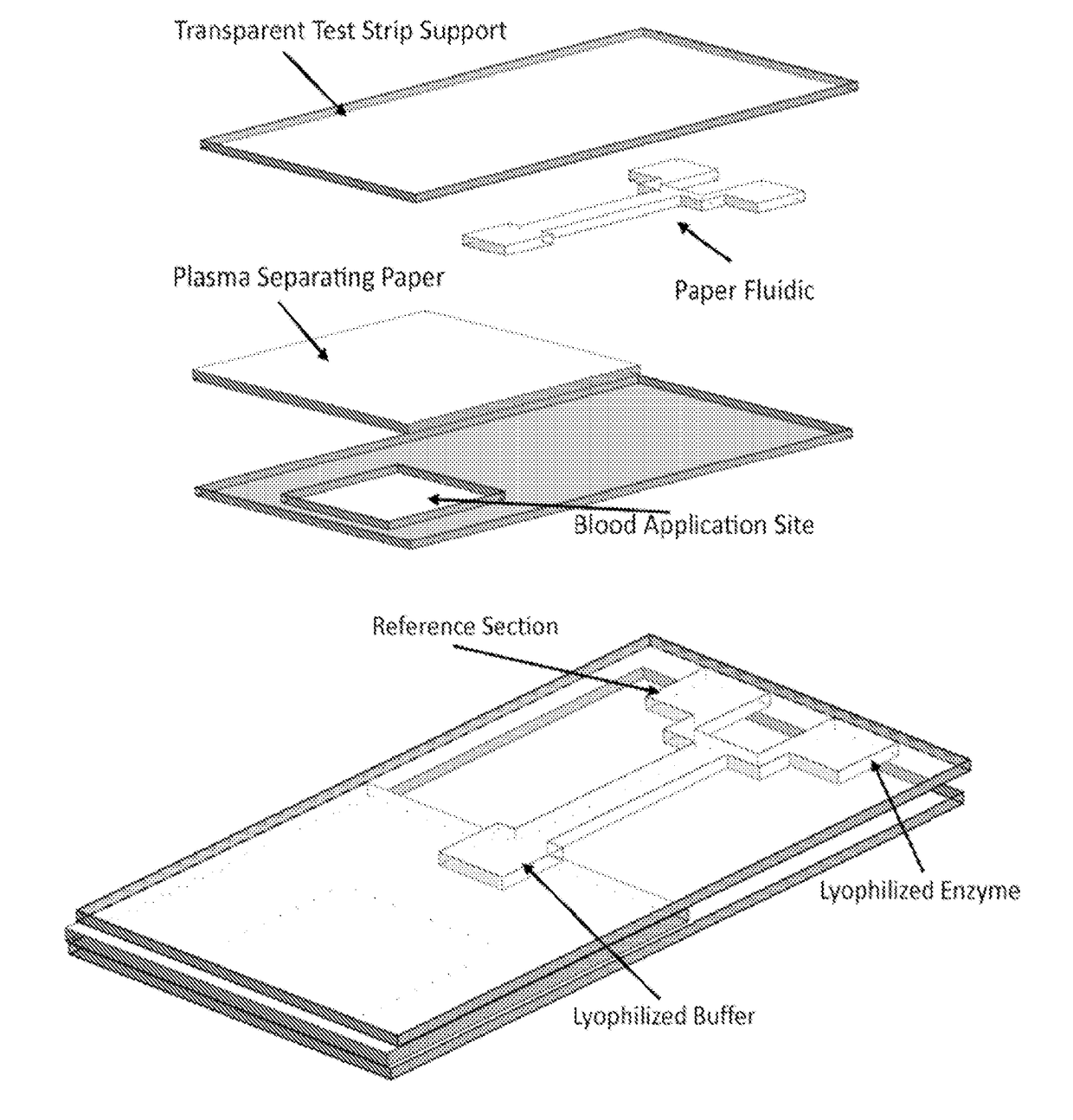
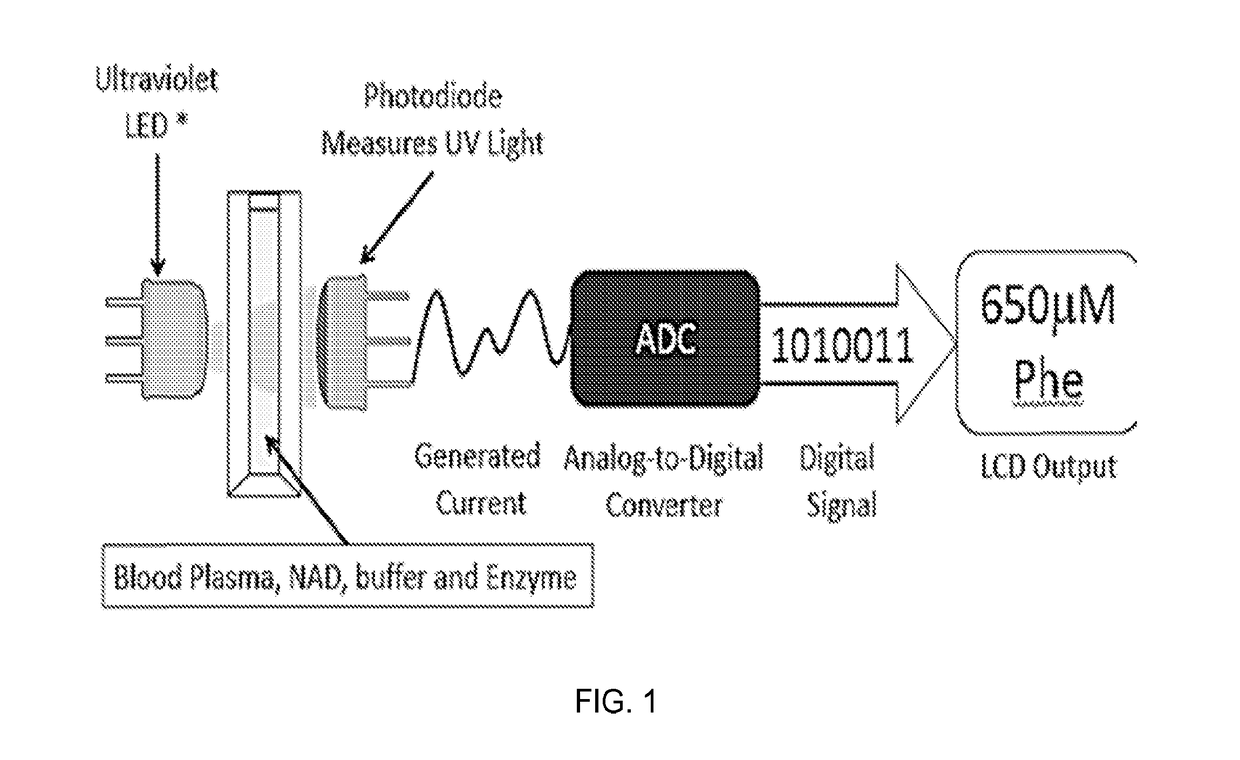
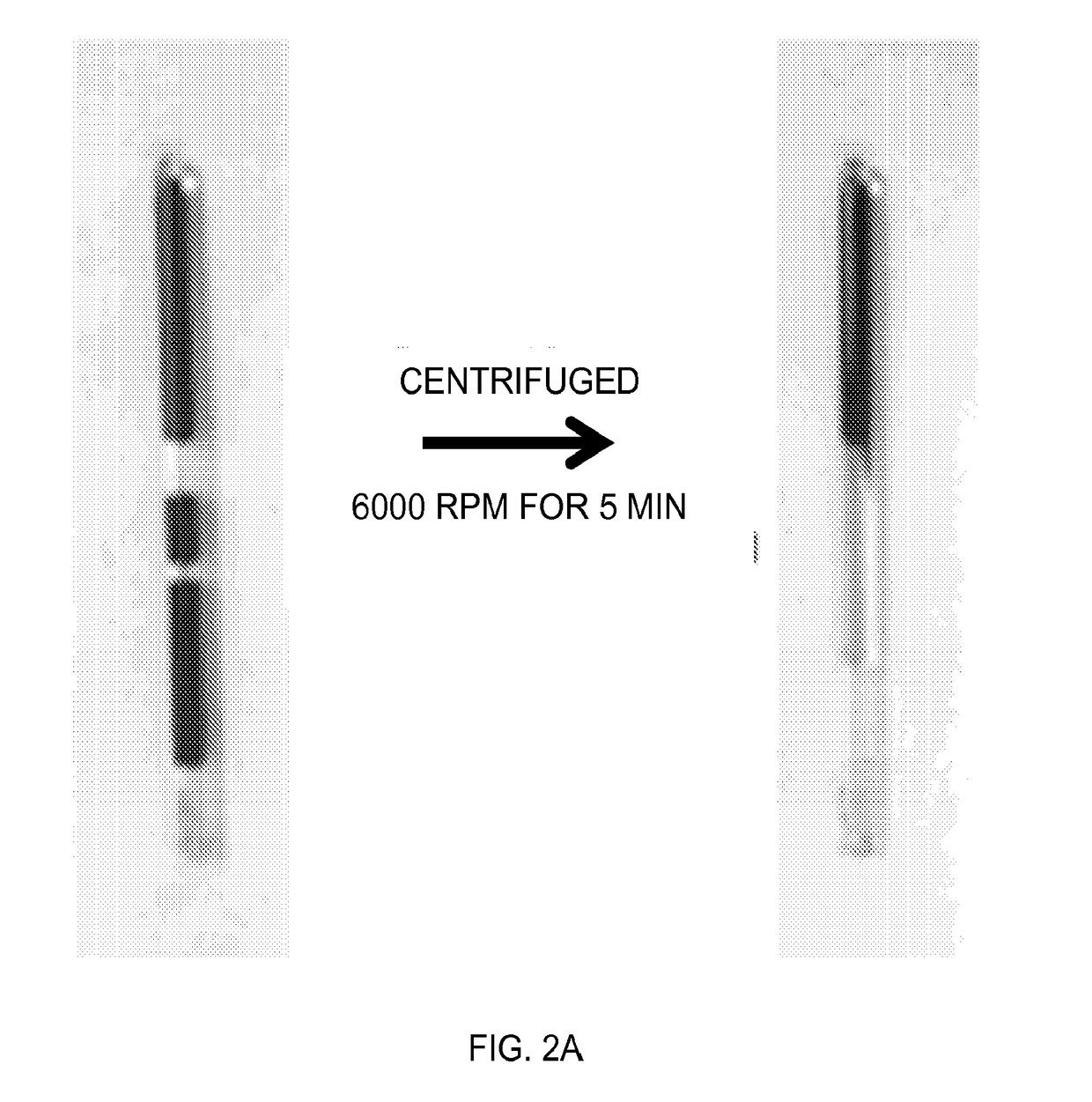
Device and methods of using device for detection of aminoacidopathies
PendingUS20170198329A1Microbiological testing/measurementLaboratory glasswaresMetabolic enzymesAmino acid
Owner:UNIV OF MARYLAND
In vitro synthesis of a layered cell sorted tissue
InactiveUS20020120950A1Easy to testAvoid spreadingBiocideEpidermal cells/skin cellsLymphatic SpreadBiology
Owner:XGENE CORP
Methods and kits for determining the occurrence of a liver disease in a subject
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Method for prediction of bone fractures by osteocalcin measurements
Owner:KAKONEN SANNA MARIA +4
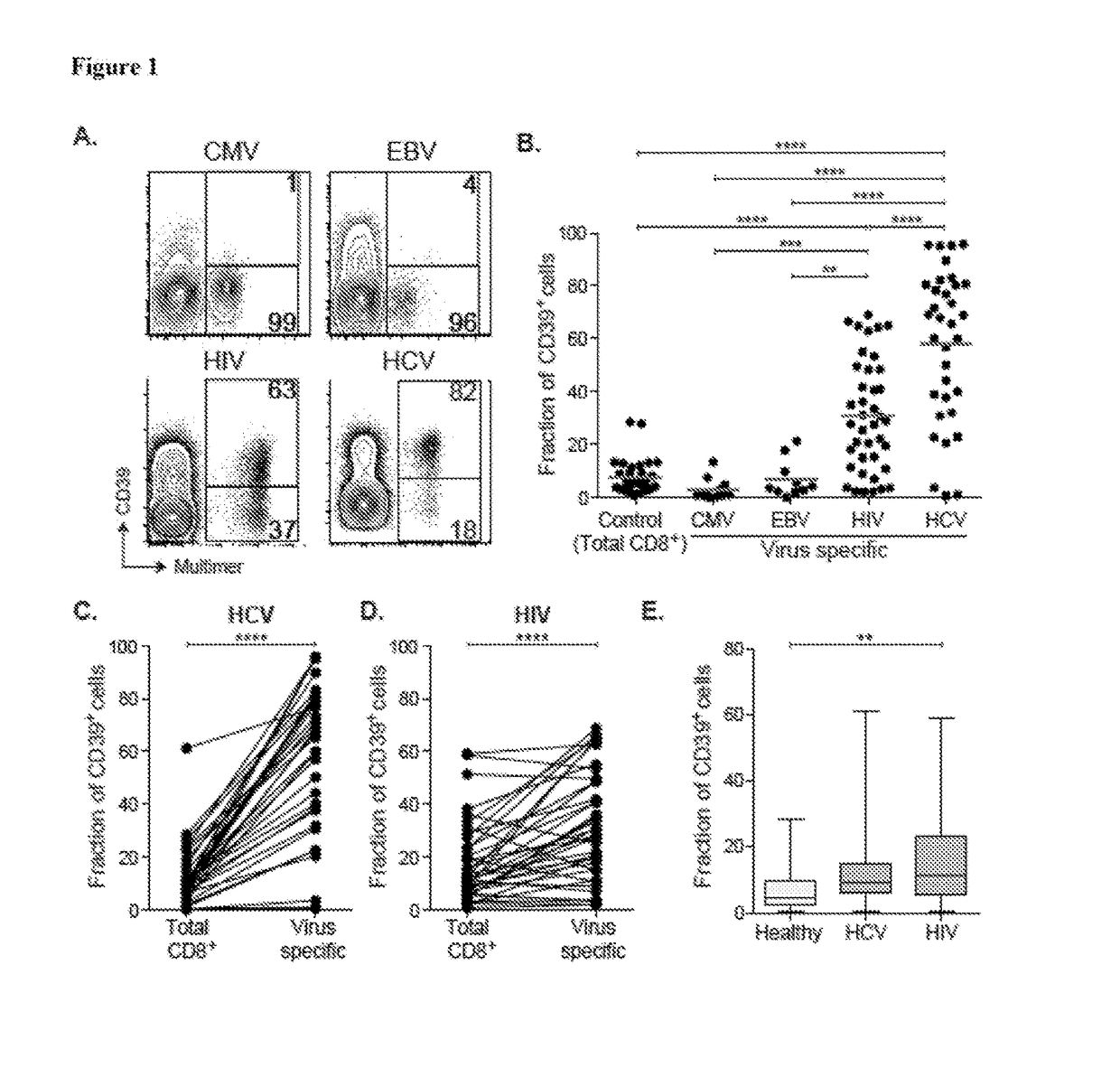
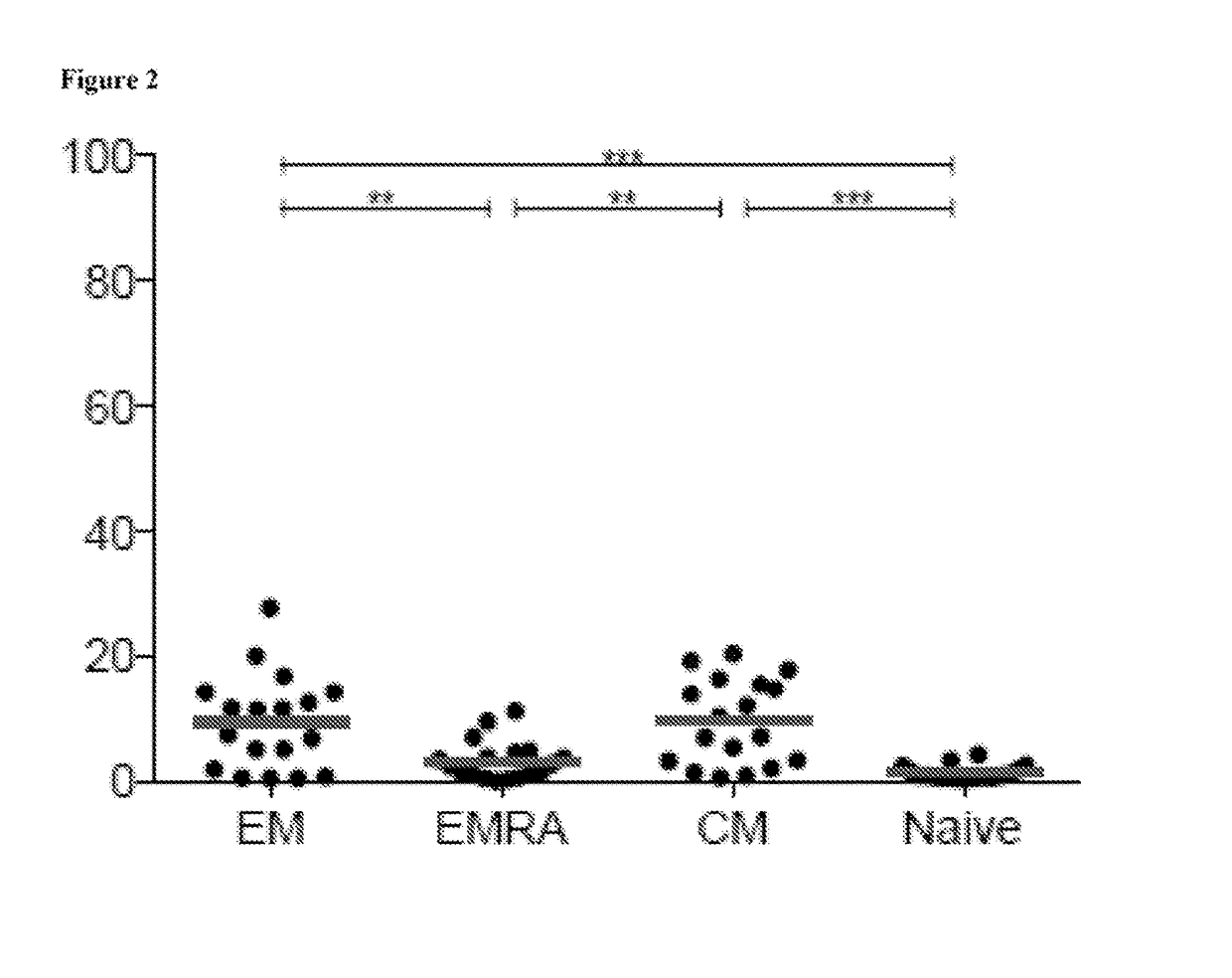
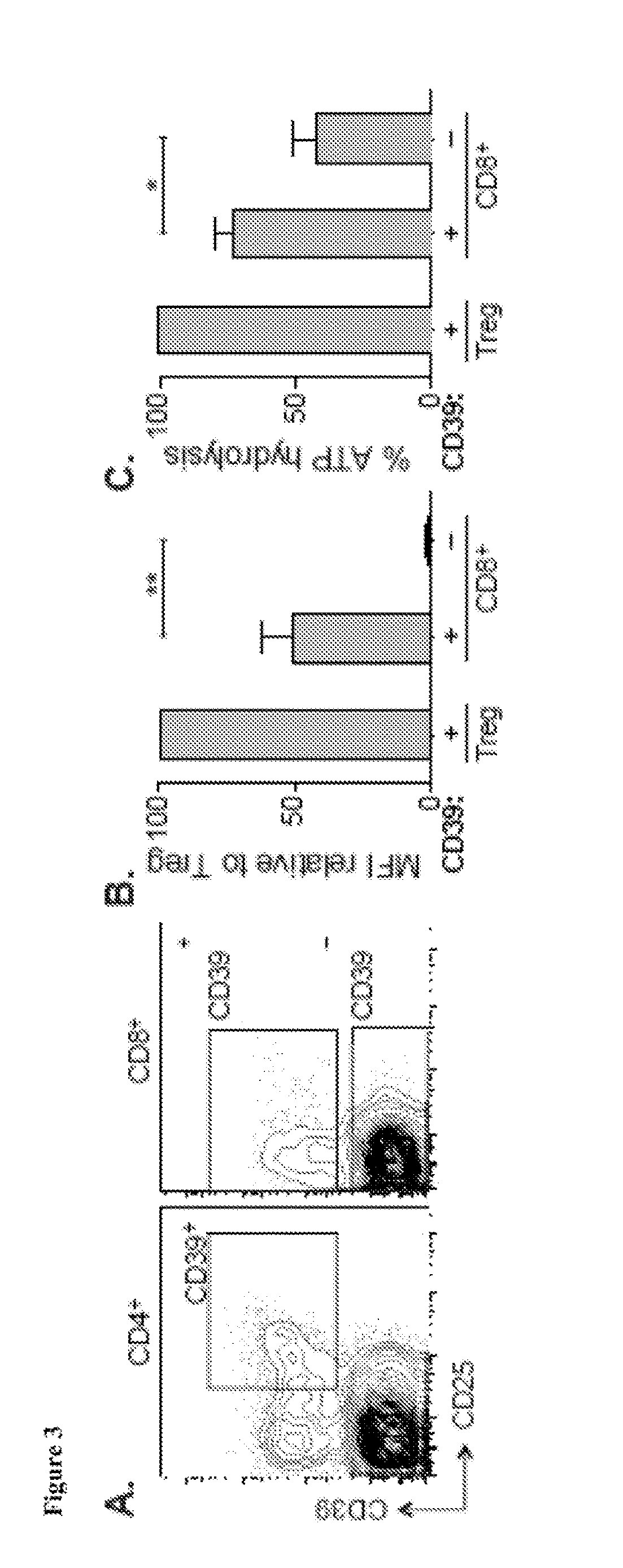
Compositions and methods for identification, assessment, prevention, and treatment of t-cell exhaustion using cd39 biomarkers and modulators
ActiveUS20170233808A1Reduces CD8+ T cell exhaustionReduce exhaustMicrobiological testing/measurementDisease diagnosisT cellInternal medicine
The present invention is based on the identification, of compositions and methods for the identification, assessment, prevention, and treatment of T-cell exhaustion using CD39 biomarkers and modulators.
Owner:DANA FARBER CANCER INST INC +1
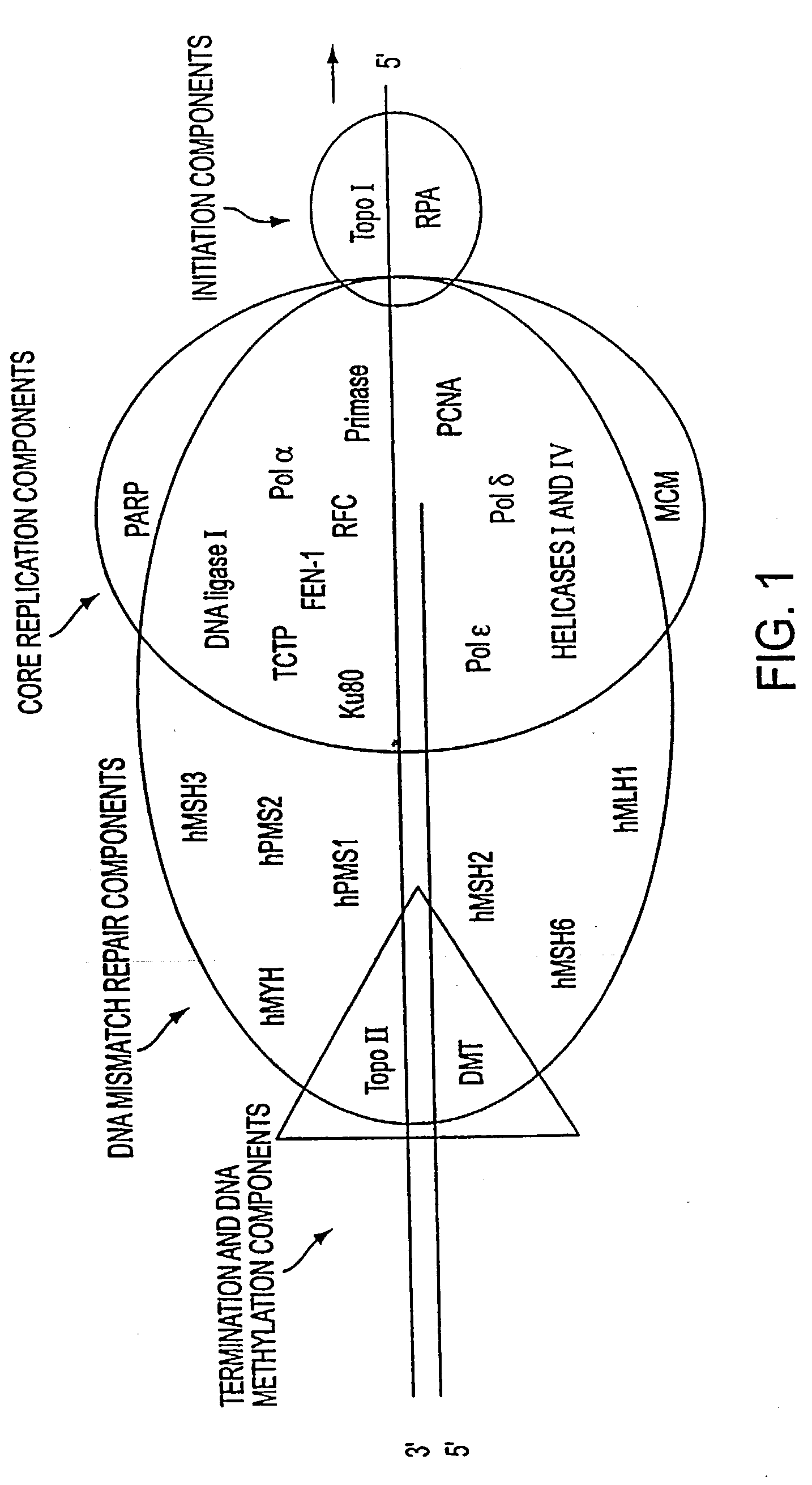
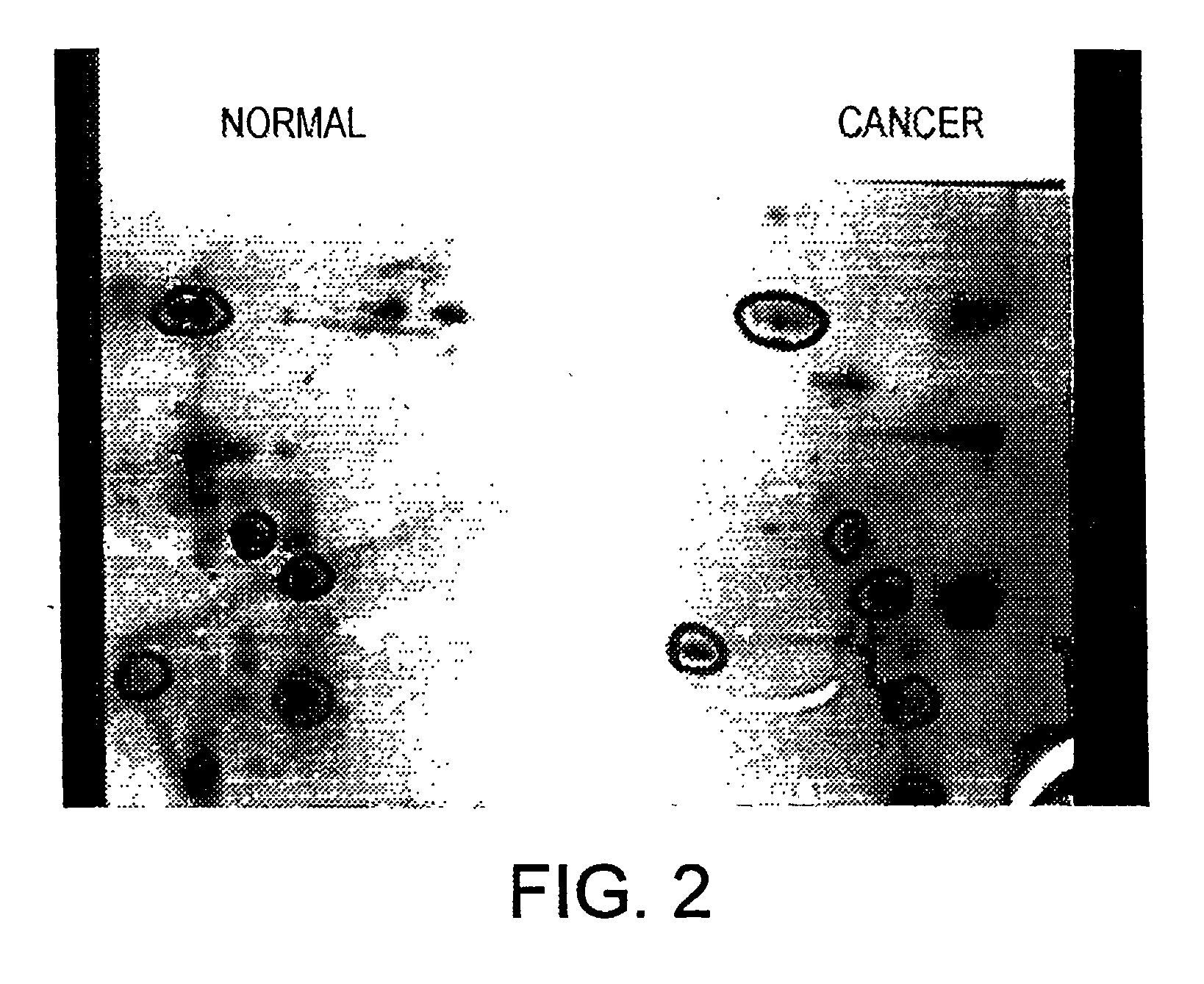
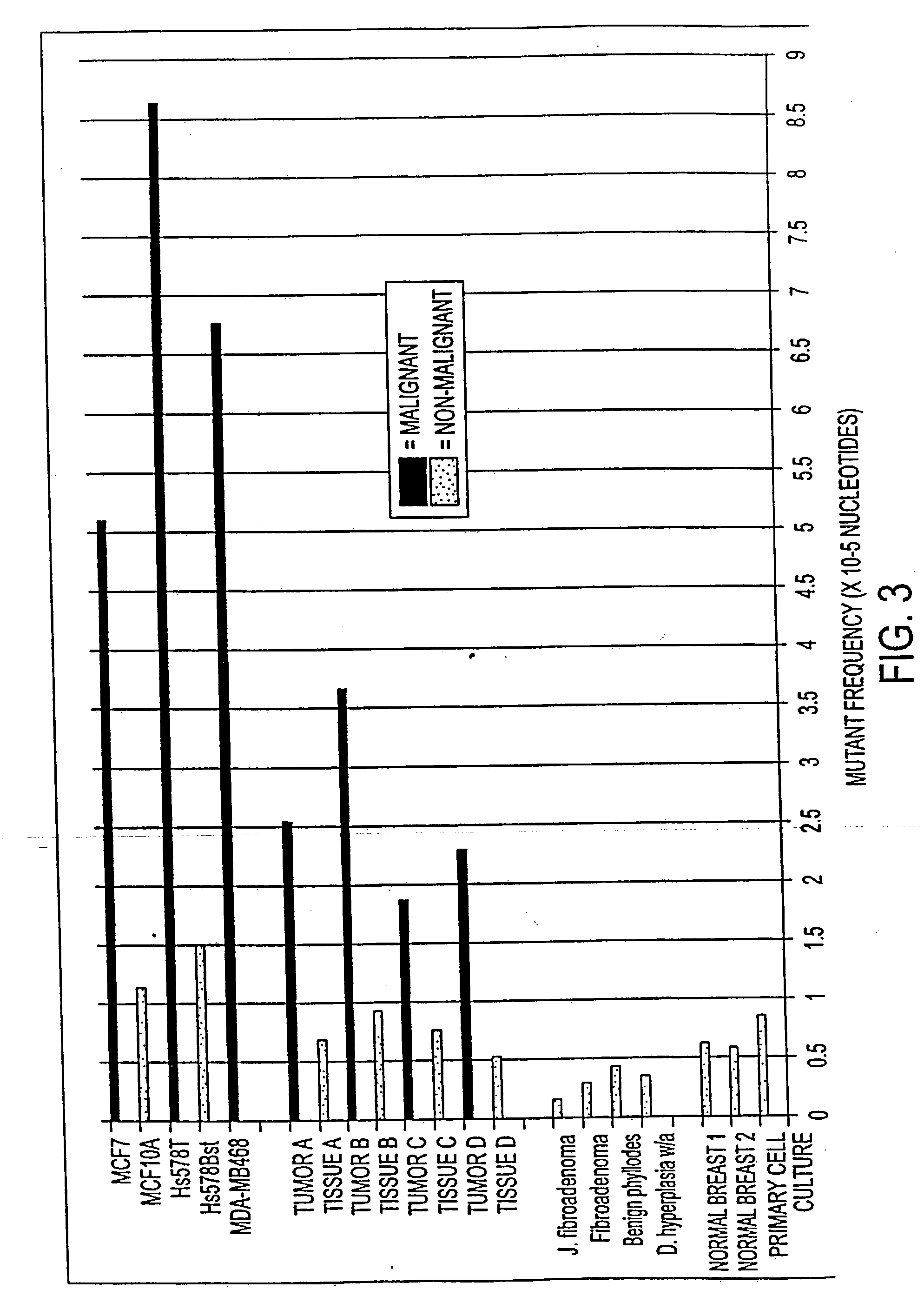
Altered DNA synthesome components as biomarkers for malignancy
InactiveUS20060073477A1Guaranteed functionChange activityPeptide/protein ingredientsMicrobiological testing/measurementMalignant phenotypeNeoplasm
Owner:SCHNAPER LAUREN
Composite quality control product of liver function, and preparation method and application thereof
ActiveCN112063686AAvoid inactivationHigh purityMicrobiological testing/measurementDisease diagnosisNucleotidaseUltrafiltration
The invention relates to a composite quality control product of a liver function, and a preparation method and application thereof, wherein the composite quality control product comprises alpha-L-fucosidase, adenosine deaminase and 5'-nucleotidase; and the preparation method comprises the following steps of: taking a fresh pork liver as a raw material, and obtaining the composite quality control product of the liver function simultaneously containing three kinds of enzymes through processes of grinding, homogenizing, crushing, centrifuging, salting-out, purifying, ultrafiltration, freeze-drying and the like. By optimizing preparation process parameters, enzyme inactivation in the preparation process is avoided; meanwhile, impurities in the finished product are reduced; therefore, the purity of the target enzyme is improved; furthermore, the freeze-dried powder of the composite quality control product with better stability is obtained through a freeze-drying process; furthermore, a protective agent of enzyme solution is optimized, so that the thermal stability and long-term stability of the freeze-dried powder of the composite quality control product are enhanced; and thus, the composite quality control product of the liver function, which is simple and rapid in preparation method, low in cost and high in enzyme activity, uniformity and stability, is obtained.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Methods for determining the risk of acute graft versus host disease
ActiveUS20150301022A1Increased riskBiocidePhosphorous compound active ingredientsCandidate donorSub populations
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
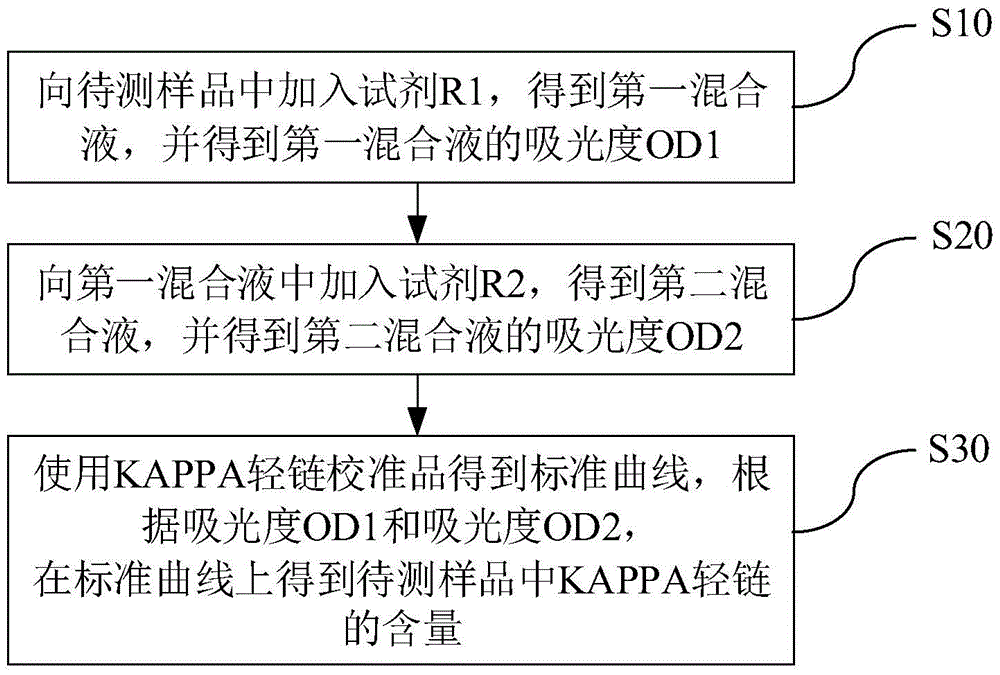
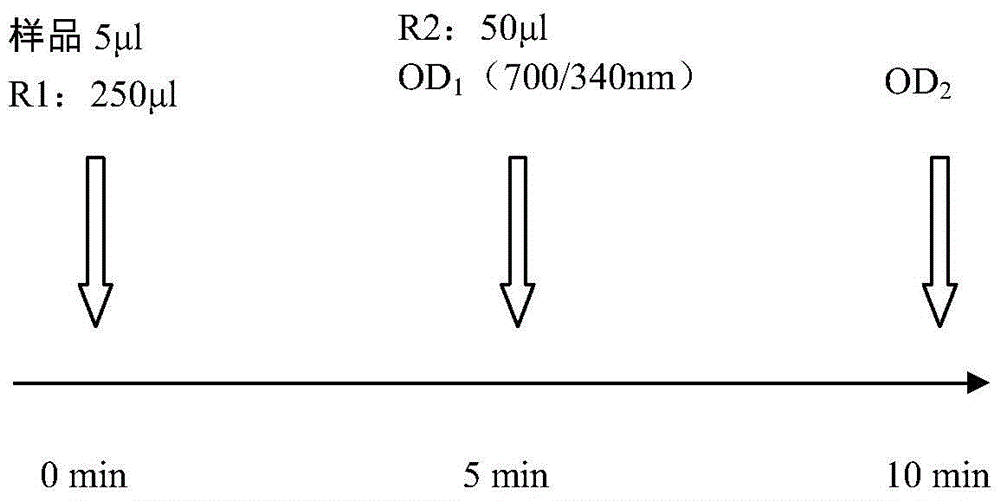
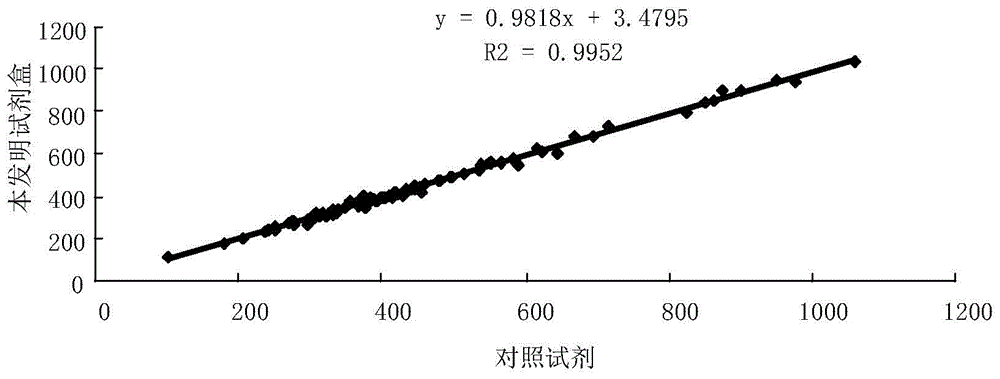
Kit and method for detecting content of KAPPA light chain and application of kit
ActiveCN105738300ADetection is simple and fastEasy to useDisease diagnosisColor/spectral properties measurementsAntigenC1-inhibitor
Owner:潍坊三维生物工程集团有限公司
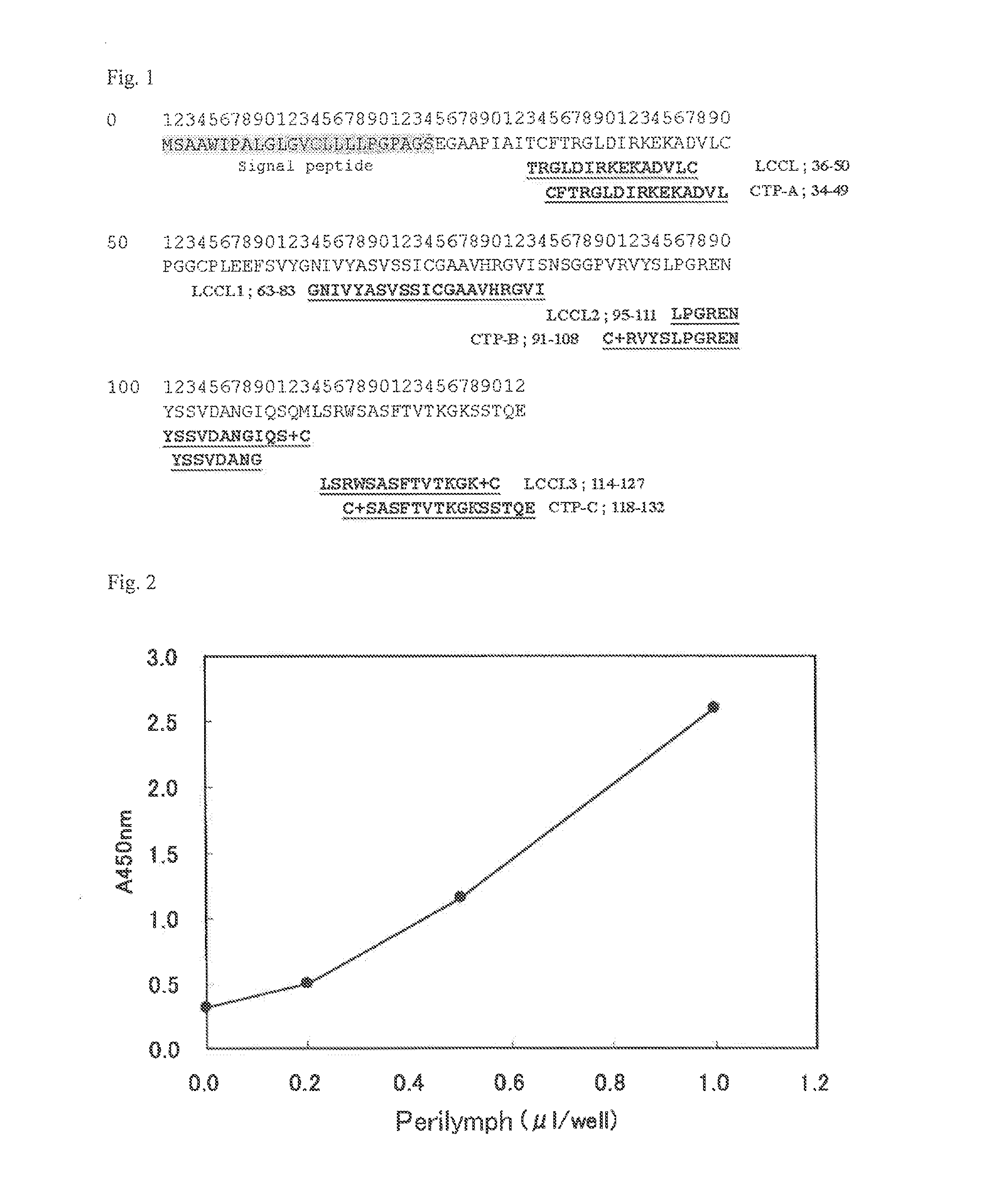
Antibody reacting with native cochlin-tomoprotein (CTP) and method for measuring ctp using same
ActiveUS20140030742A1Accurate and Rapid DiagnosisSimple procedureImmunoglobulins against animals/humansDisease diagnosisAntigenAmino acid
Owner:SAITAMA MEDICAL UNIVERSITY
Diagnostic and immunotherapy compositions and methods for disease states mediated by inhibitor-resistant cd8 t-cells
Owner:JOHNSON RAYMOND M
Electrochemical immunosensor of electroactive substance modified MOF composite material as well as preparation and application of electrochemical immunosensor
Owner:SHANTOU UNIV
Detection method of antiphospholipid syndrome related antibody
PendingCN113358864AResolve detectionResolutionDisease diagnosisIndividual particle analysisPhospholipid antibodyPhospholipin
Owner:贵州安康医学检验中心有限公司
Methods for determining the risk of a systemic lupus erythematosus (SLE) patient to develop neuropsychiatric syndromes
InactiveUS20180231565A1Successfully distinguishHighly sensitive, specific, reliable, accurate and discriminatoryBiostatisticsDisease diagnosisPediatricsDiscoid lupus erythematosus
Methods and kits are provided for diagnosing of neuropsychiatric syndromes concurrent with SLE (NPSLE) and for determining whether an SLE subject is at risk of developing a neuropsychiatric disease.
Owner:IMMUNARRAY LTD
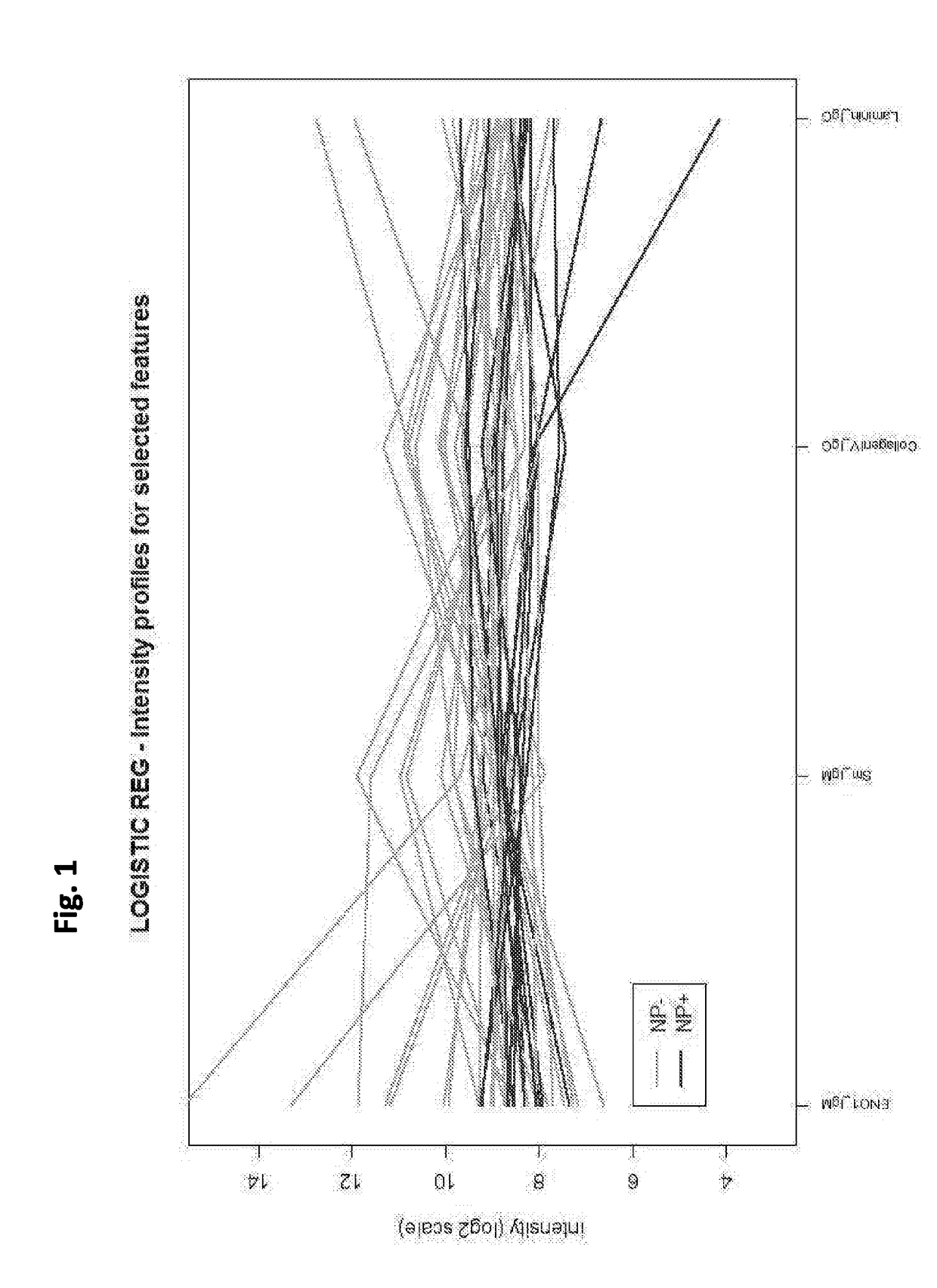
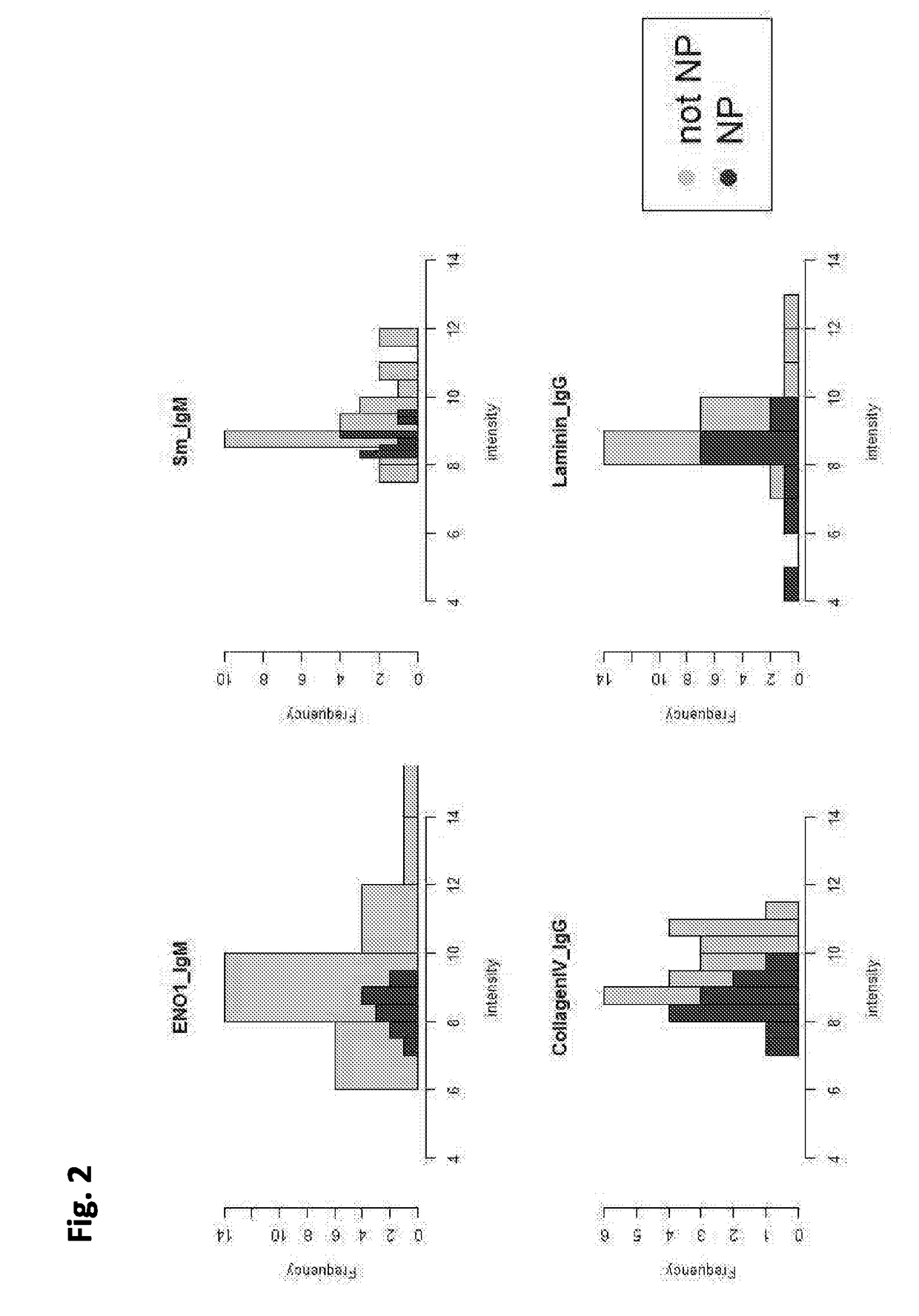
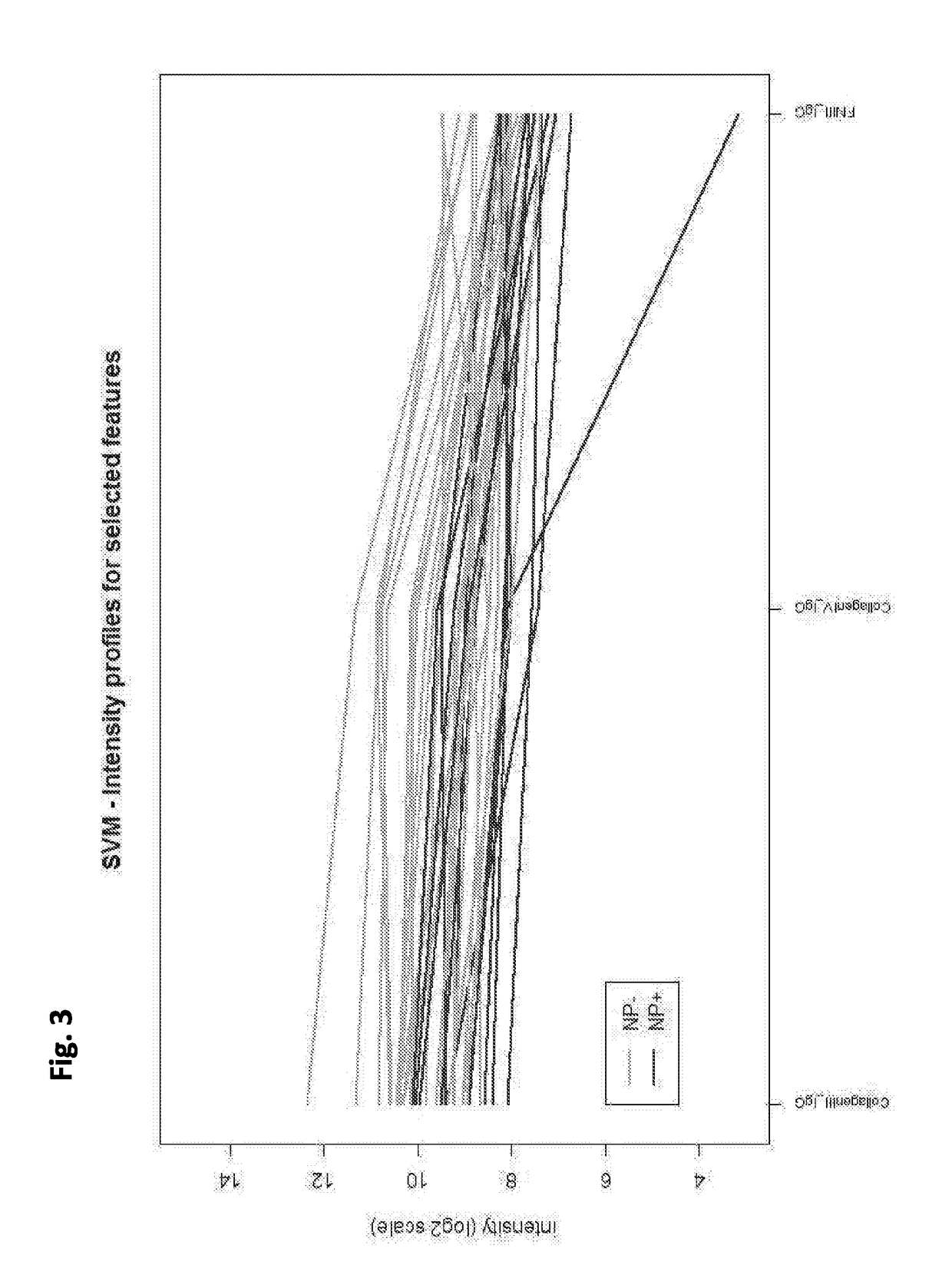
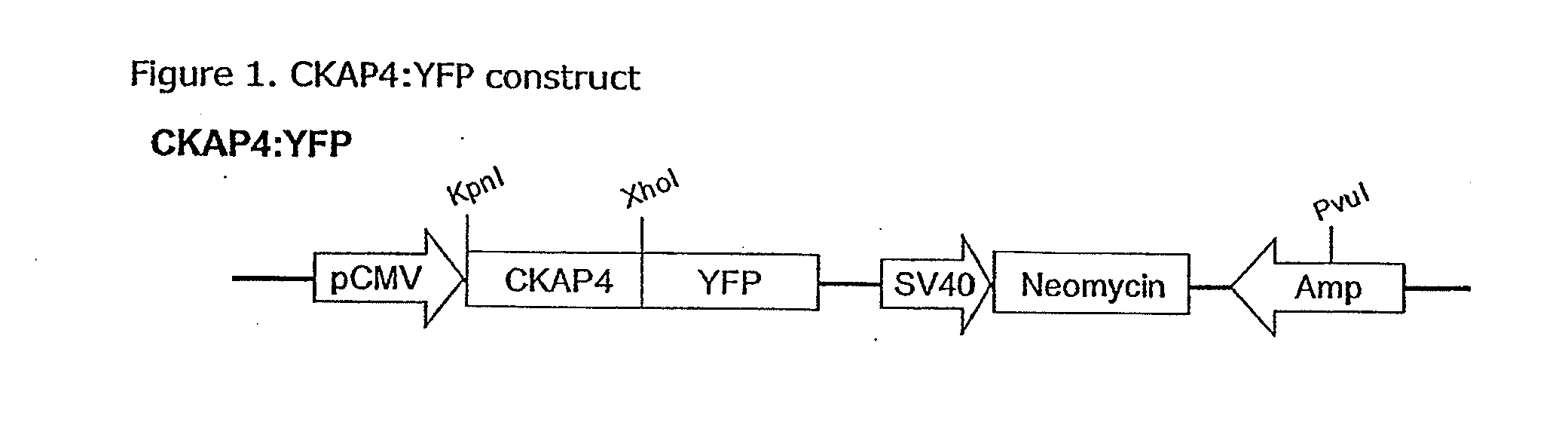
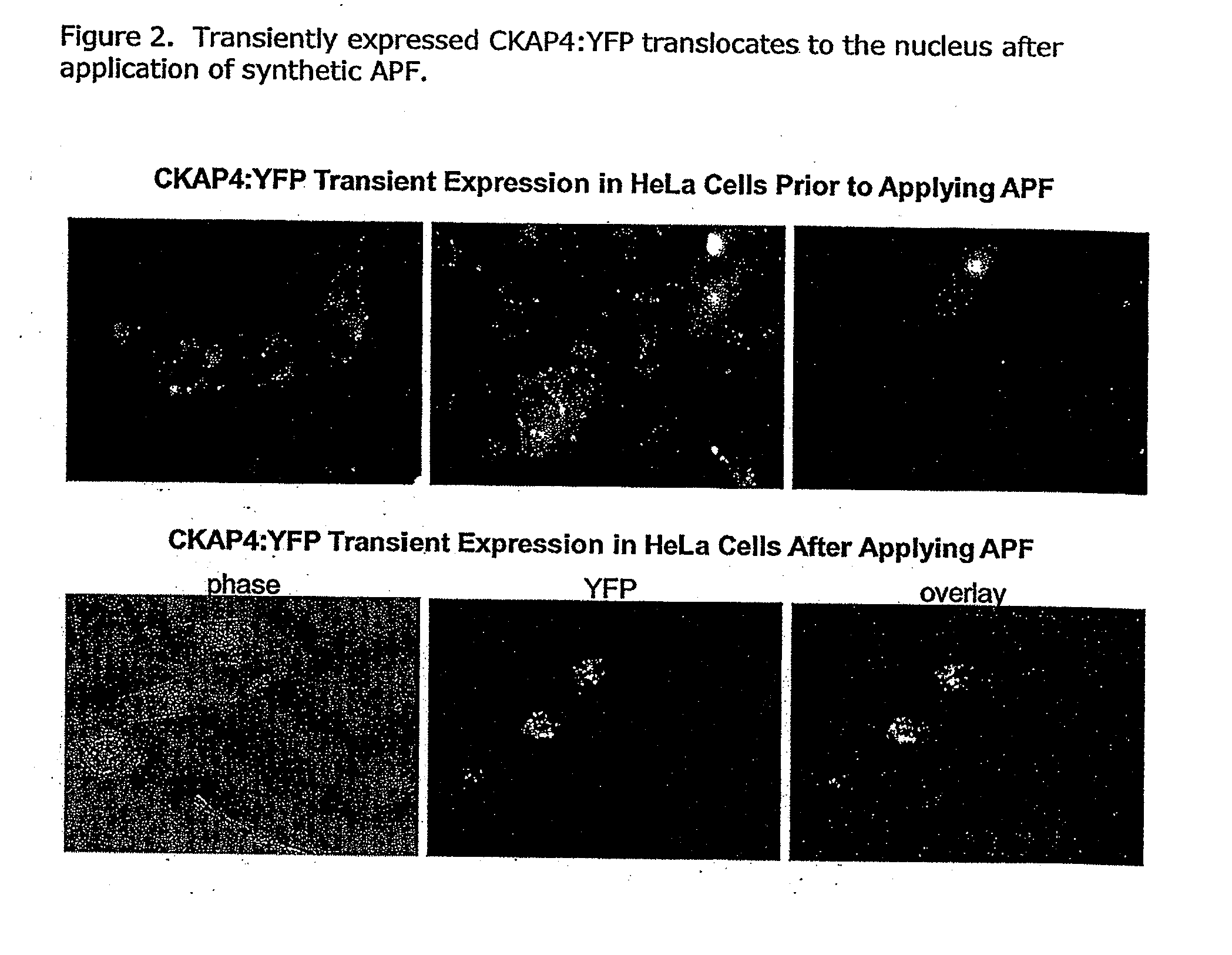
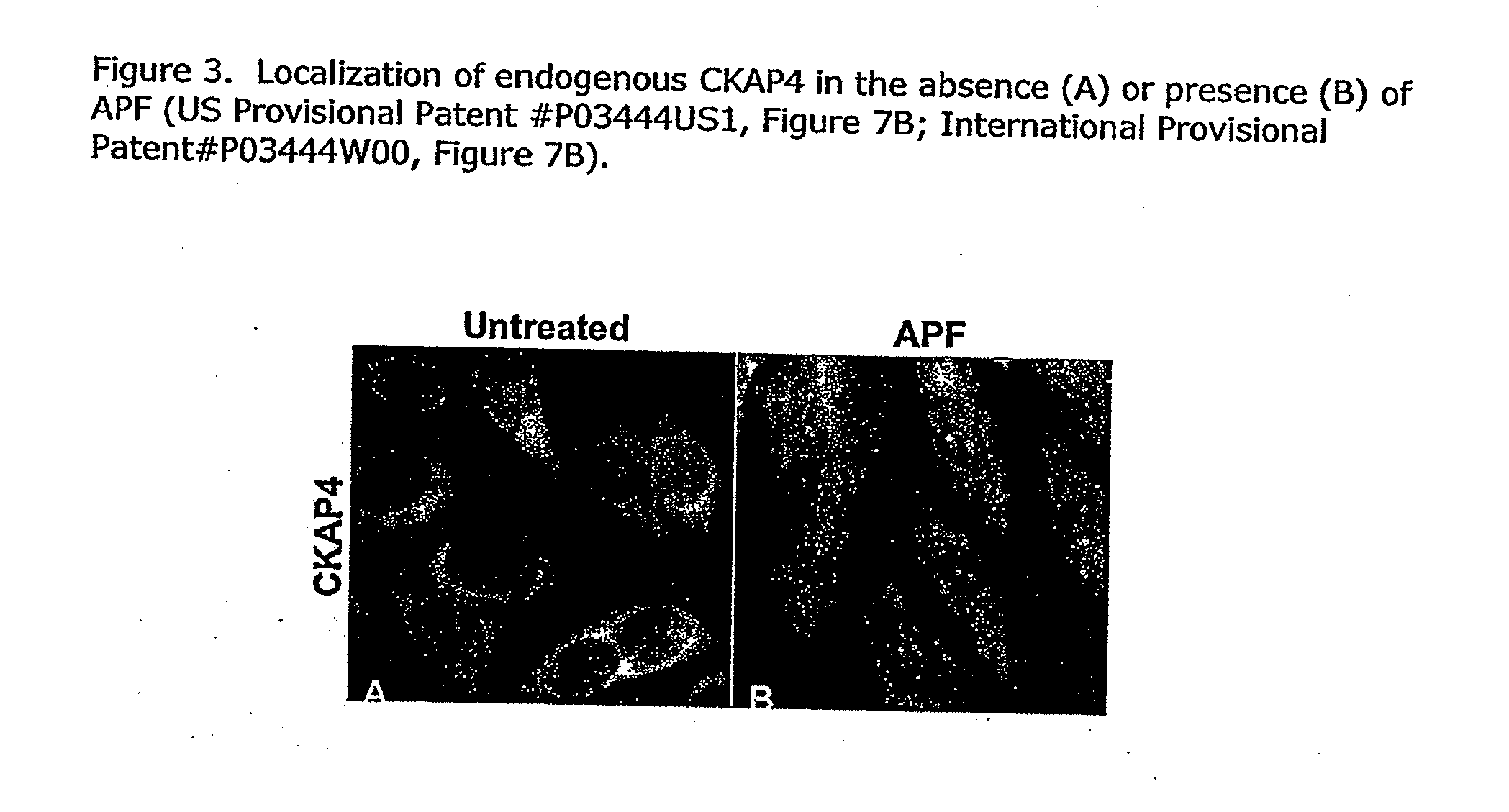
Cell-based detection of apf through its interaction with ckap4 for diagnosis of interstitial cystitis
ActiveUS20110244493A1Reduced level of bindingInhibit bindingCell receptors/surface-antigens/surface-determinantsAnalysis using chemical indicatorsSystems designInterstitial cystitis
Owner:THE COMMONWEALTH MEDICAL COLLEGE +1
Nt-probnp/troponin ratio for assessing myocardial dysfunction
Owner:ROCHE DIAGNOSTICS OPERATIONS
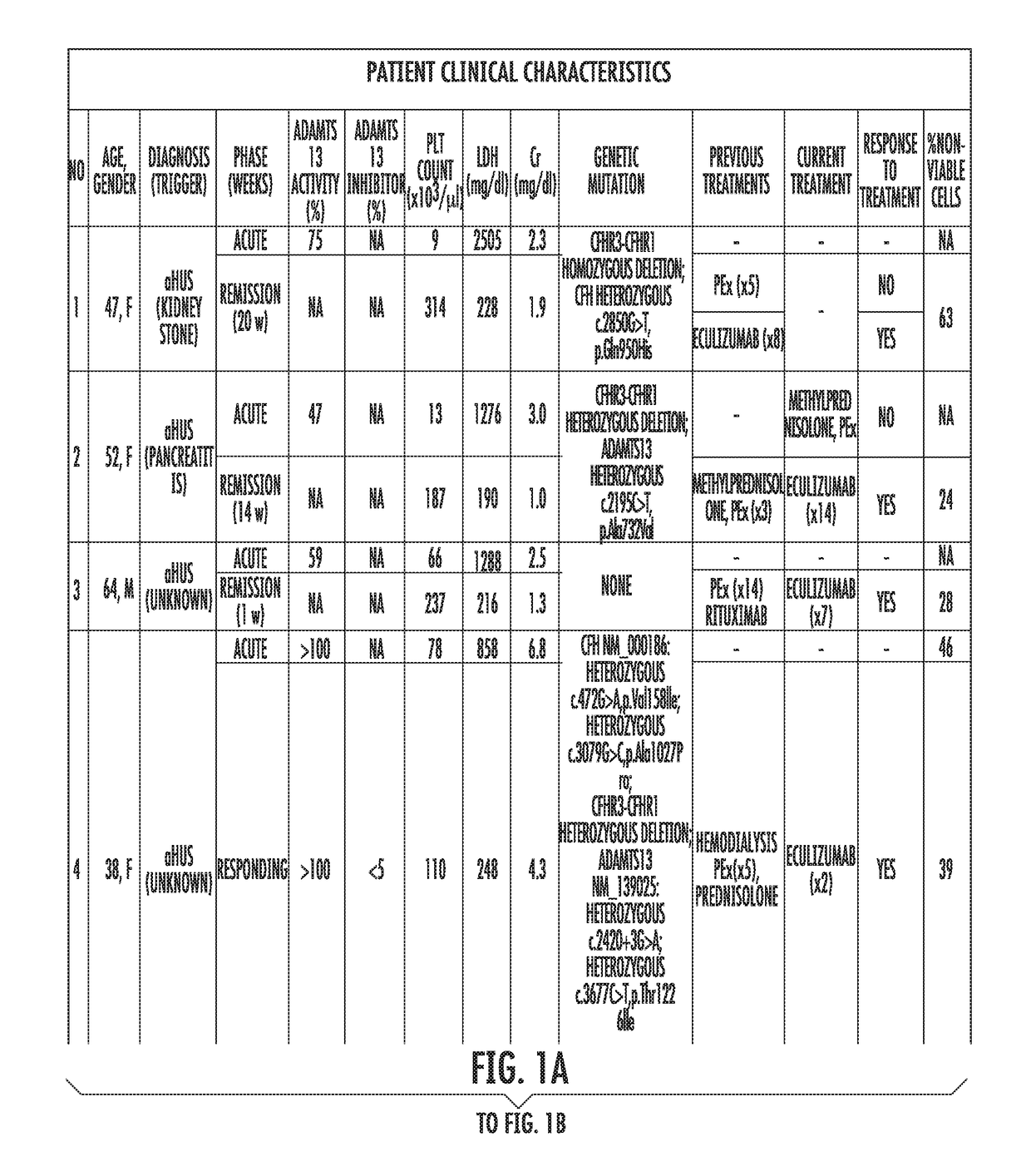
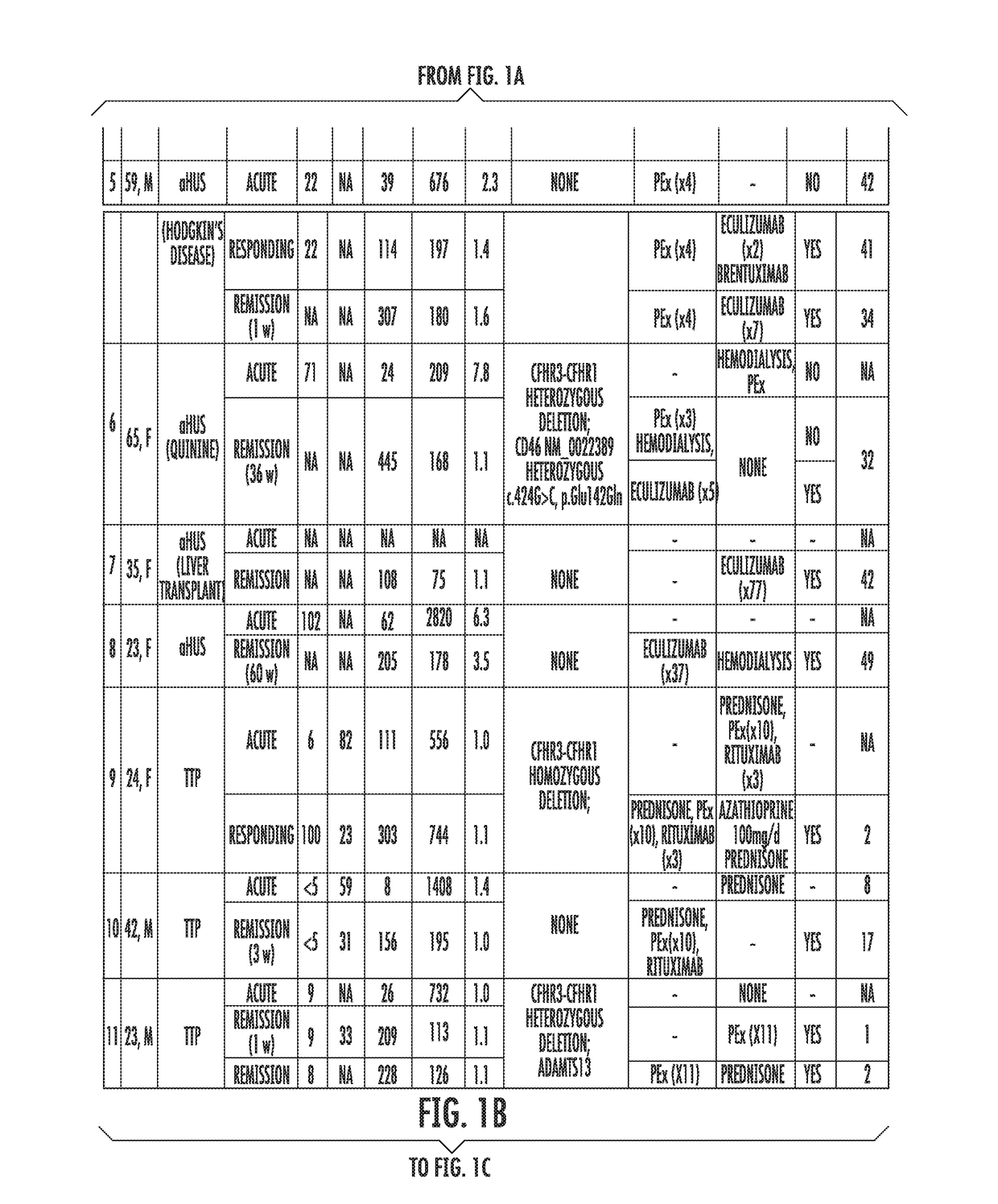
Assay to diagnose and treat disorders of the alternative pathway of complement activation
ActiveUS20180246082A1Simple and rapid and inexpensive assayIncreased activationImmunoglobulins against animals/humansDisease diagnosisBlood serumViable cell
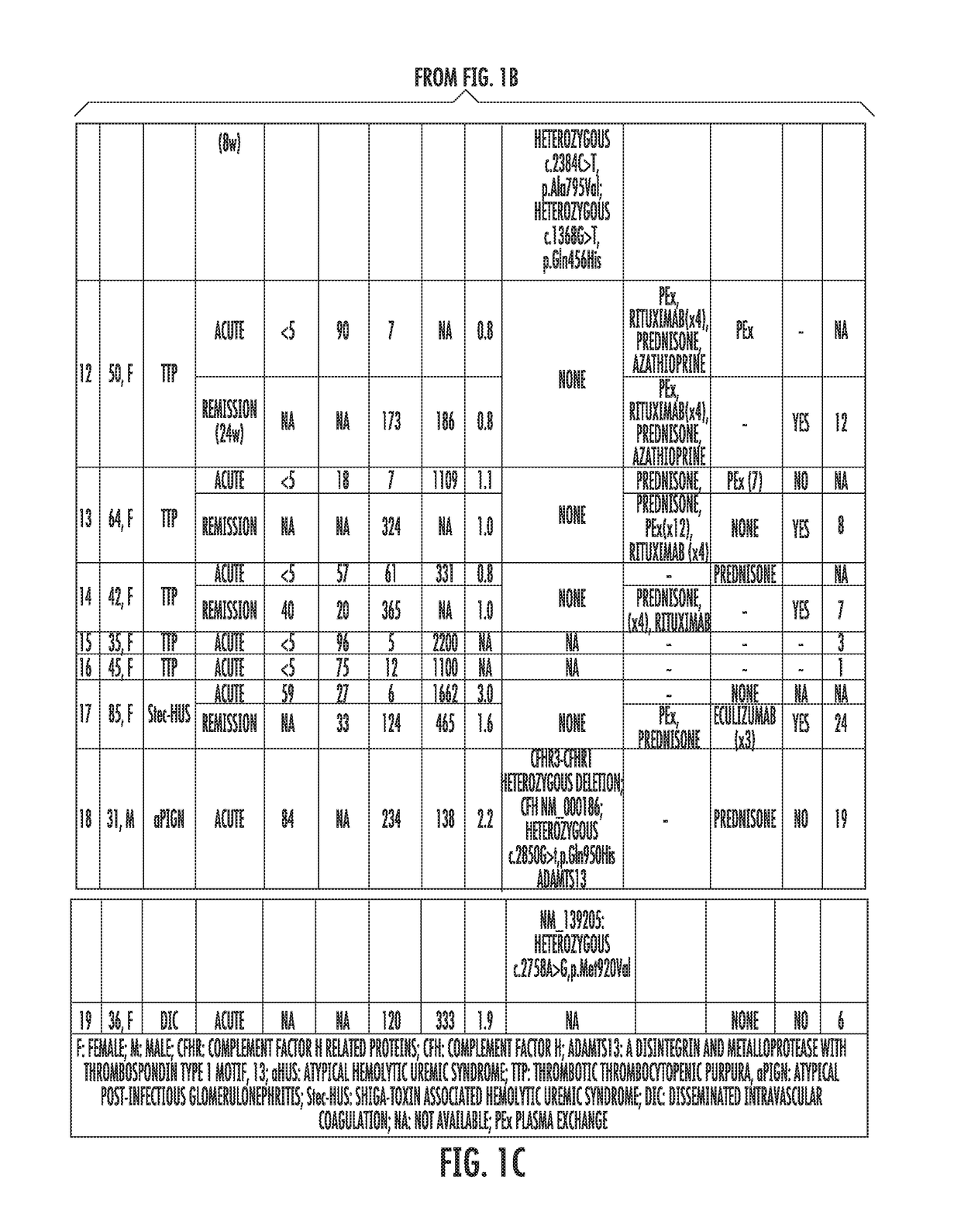
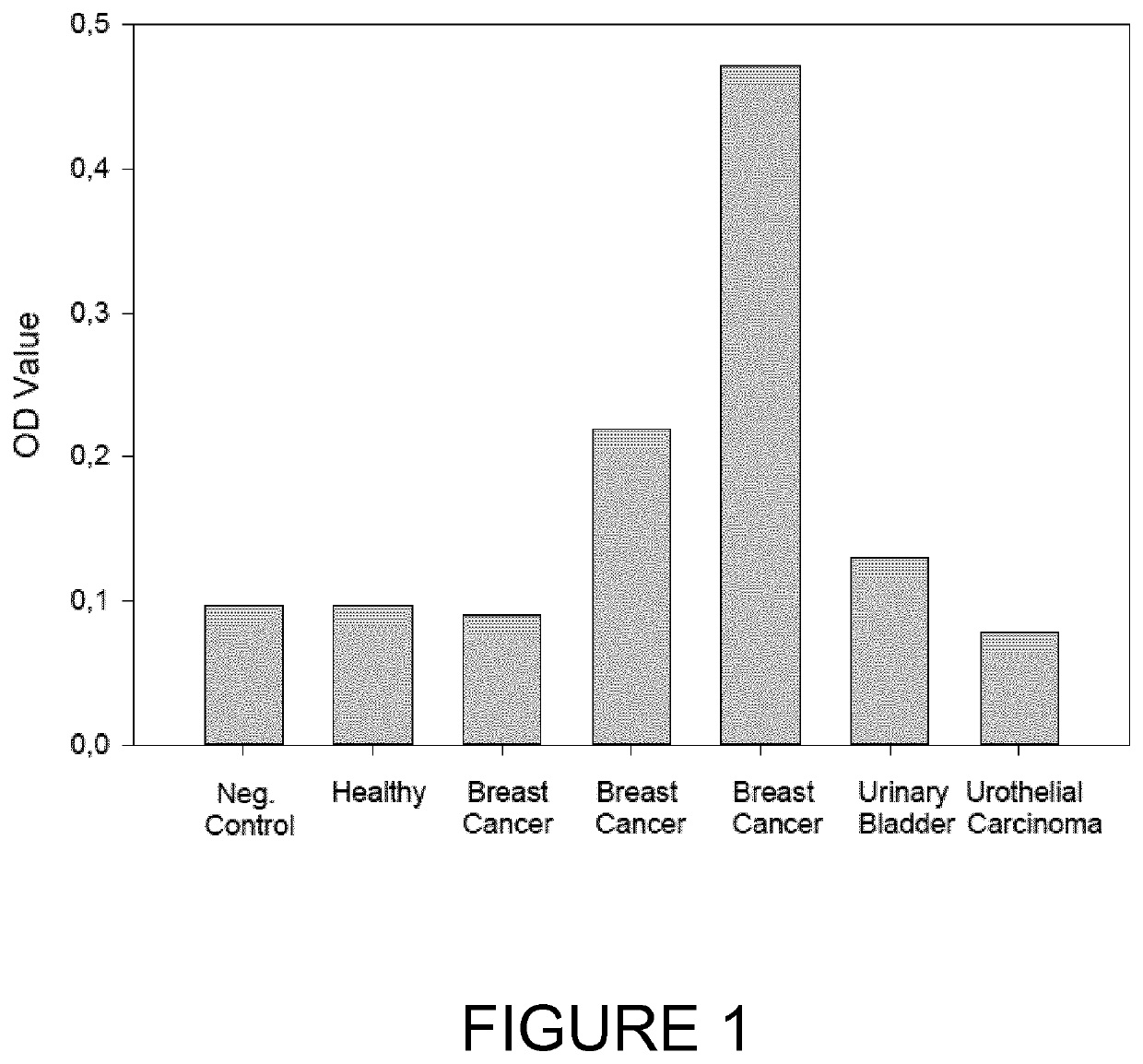
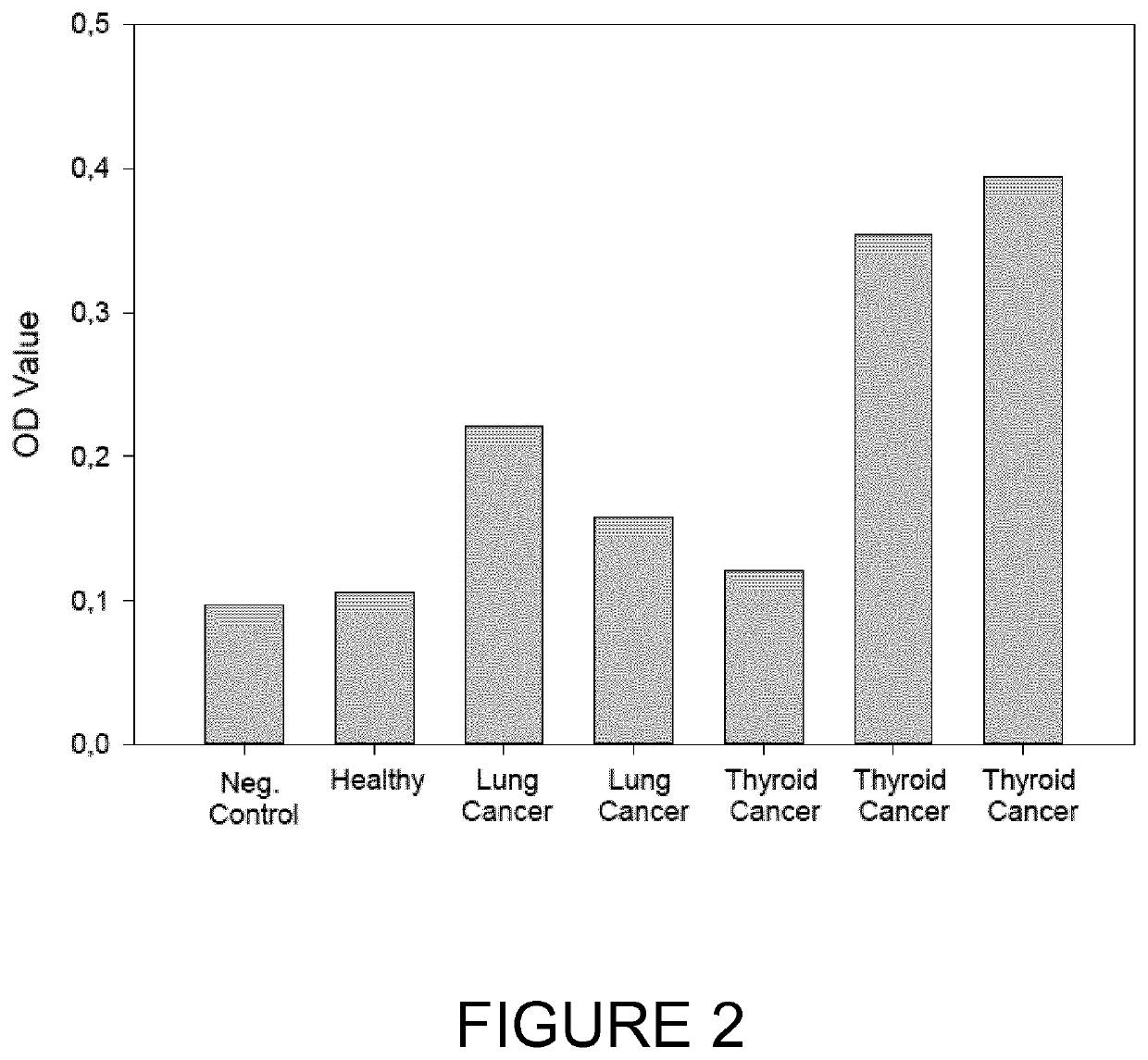
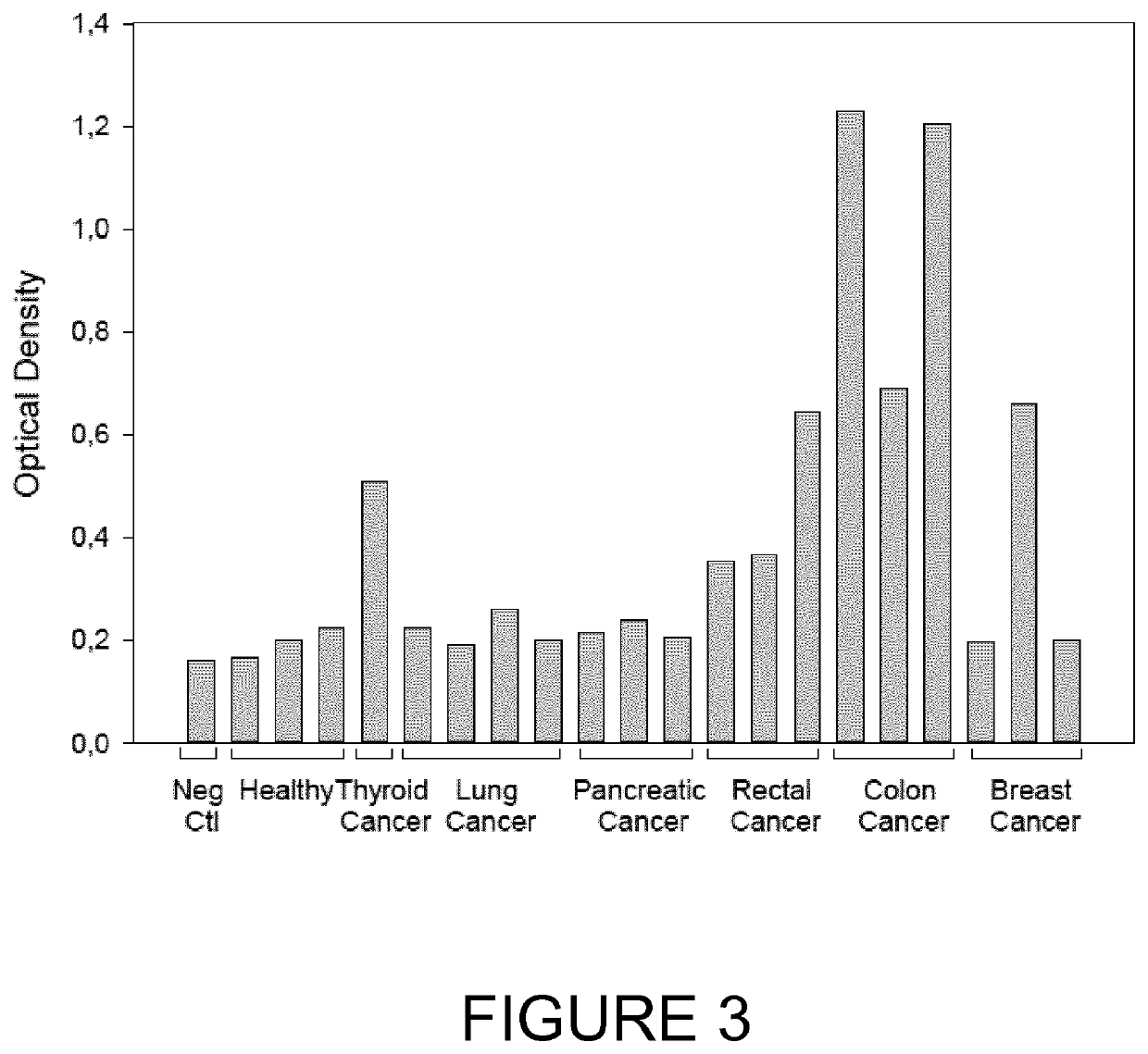
The present invention relates generally to the field of disorders of complement activation. More specifically, the present invention provides methods and compositions useful for diagnosing and treating atypical hemolytic uremic syndrome, antiphospholipid antibody syndrome and other disorders of the alternative pathway of complement activation. In one embodiment, a method comprises the steps of (a) incubating or contacting serum obtained from a patient suspected of having atypical hemolytic uremic syndrome (aHUS) with a glycosylphosphatidylinositol-anchored protein (GPI-AP) deficient cell line; and (b) performing a cell viability assay on the cells from step (a). In a specific embodiment, the method further comprises the step of diagnosing the patient as having aHUS based on a statistically significant increased difference of non-viable cells from the patient serum as compared to a control.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Protein expression-based classifier for prediction of recurrence in adenocarcinoma
A method for making a prognosis for a patient afflicted with a type of cancer which comprises (a) determining in a sample of a tumor from the patient a level of expression for at least three biomarkers from the following group: thyroid transcription factor-1 (TTF1), signal transducer and activator of transcription-3 (STAT-3), beta-catenin and cyclin D1; (b) calculating a score based on the levels of expression determined in step (a); (c) correlating the score obtained in step (b) with a series of predetermined reference scores associated with a series of prognoses; so as to thereby make a prognosis for the patient.
Owner:YALE UNIV
Use of nucleosome-transcription factor complexes for cancer detection
InactiveUS20200363418A1Disease diagnosisAntineoplastic agentsTranscription factor complexBiologic marker
Owner:BELGIAN VOLITION SPRL
Preparation method of magnetic particles connected with dsDNA
ActiveCN112881680AHigh reactivityImprove efficiencyDisease diagnosisAminopyridinesCondensed matter physics
Owner:SHENZHEN DRAWRAY BIOTECH CO LTD
Preparation method and application of fluorescent reagent strip for quantitatively detecting concentration of ProAKAP4
PendingCN112630437ARealize quantitative detection of single servingReduce testing costsDisease diagnosisBiological testingReagent stripSemen
Owner:普迪特泰州生物科技有限公司
Application of MYH9 in preparation of diagnostic reagent for severe oligoasthenozoospermia and asthenozoospermia
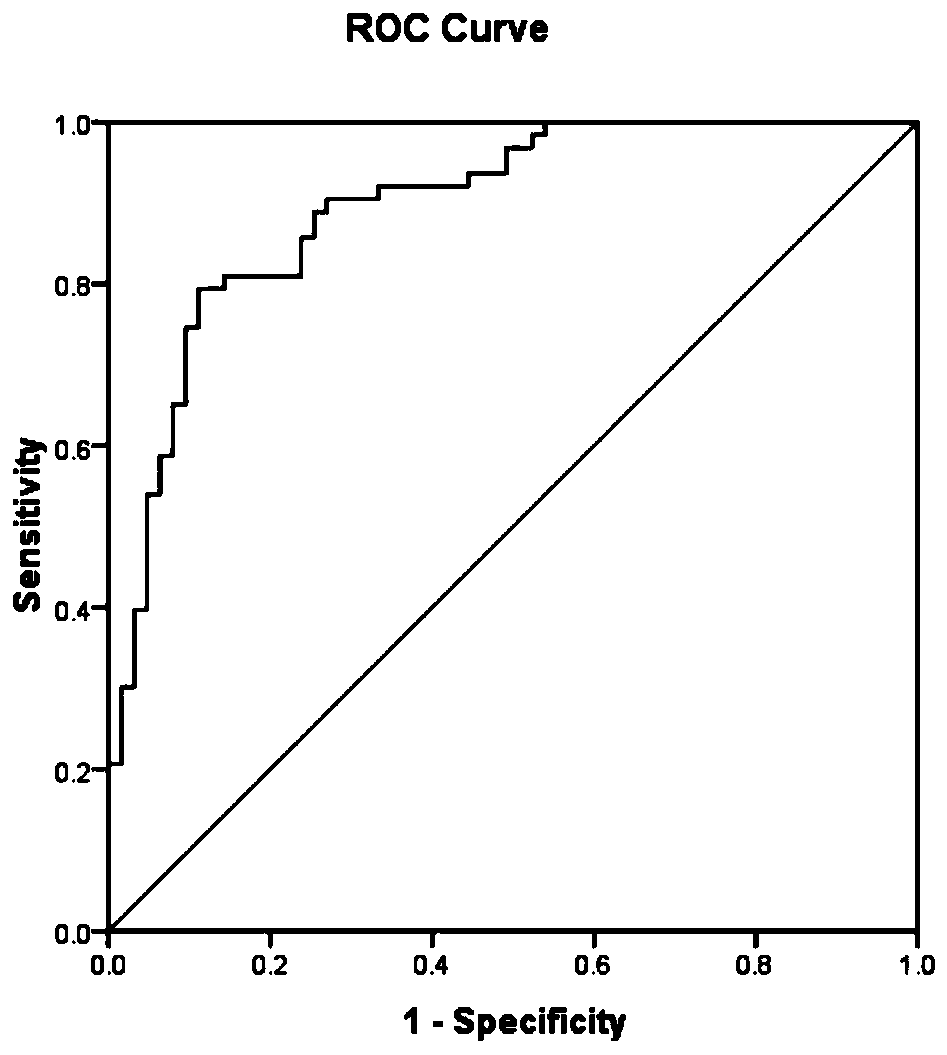
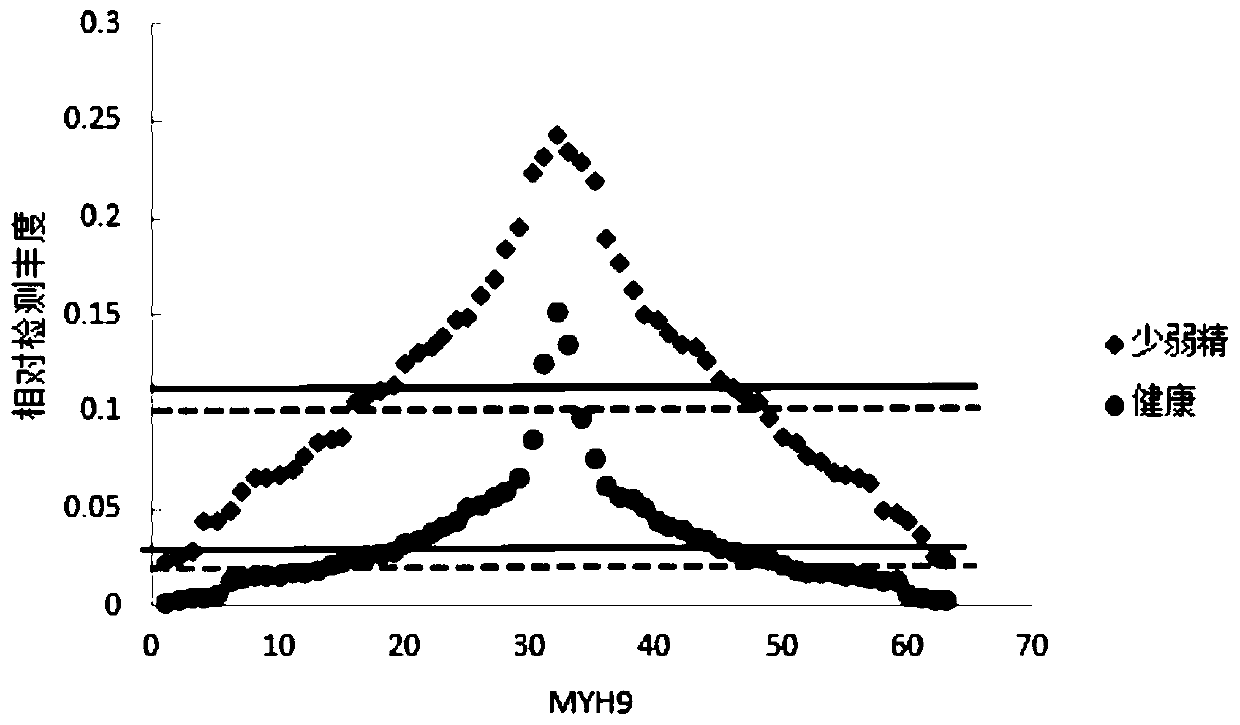
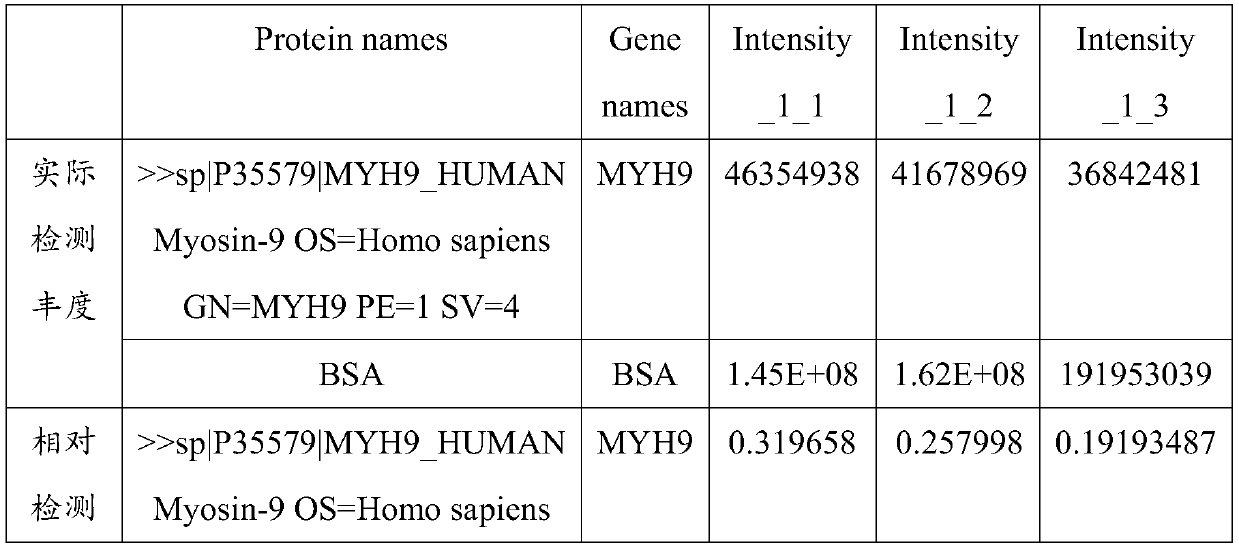
PendingCN111537749AGood correlationDisease diagnosisBiological testingProteomics methodsBiochemistry
Owner:山东立菲生物产业有限公司
Method for assisting diagnosis of risk of progression to nephropathy and use of reagent kit
Owner:SYSMEX CORP
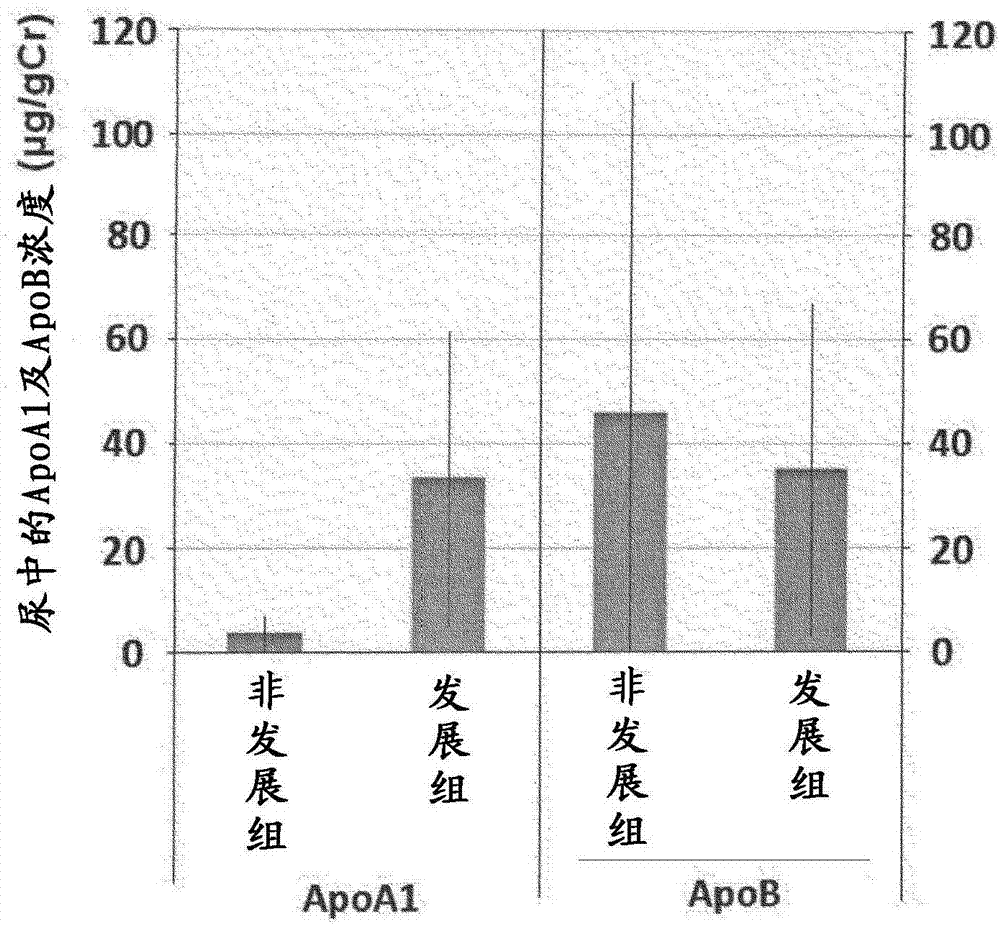
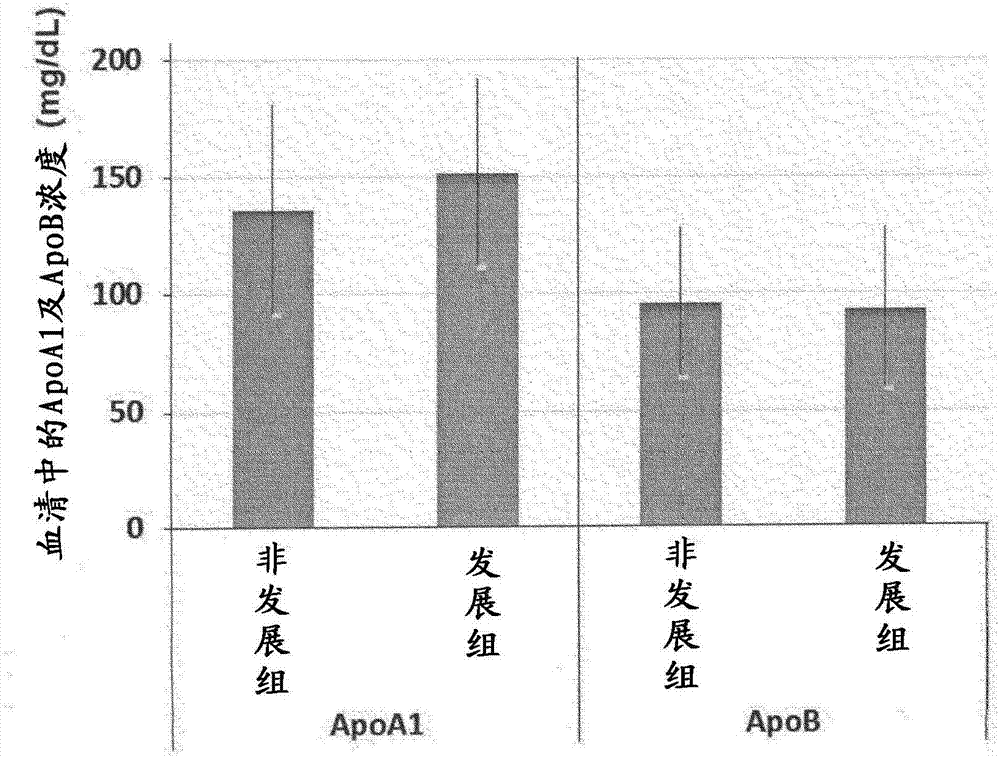
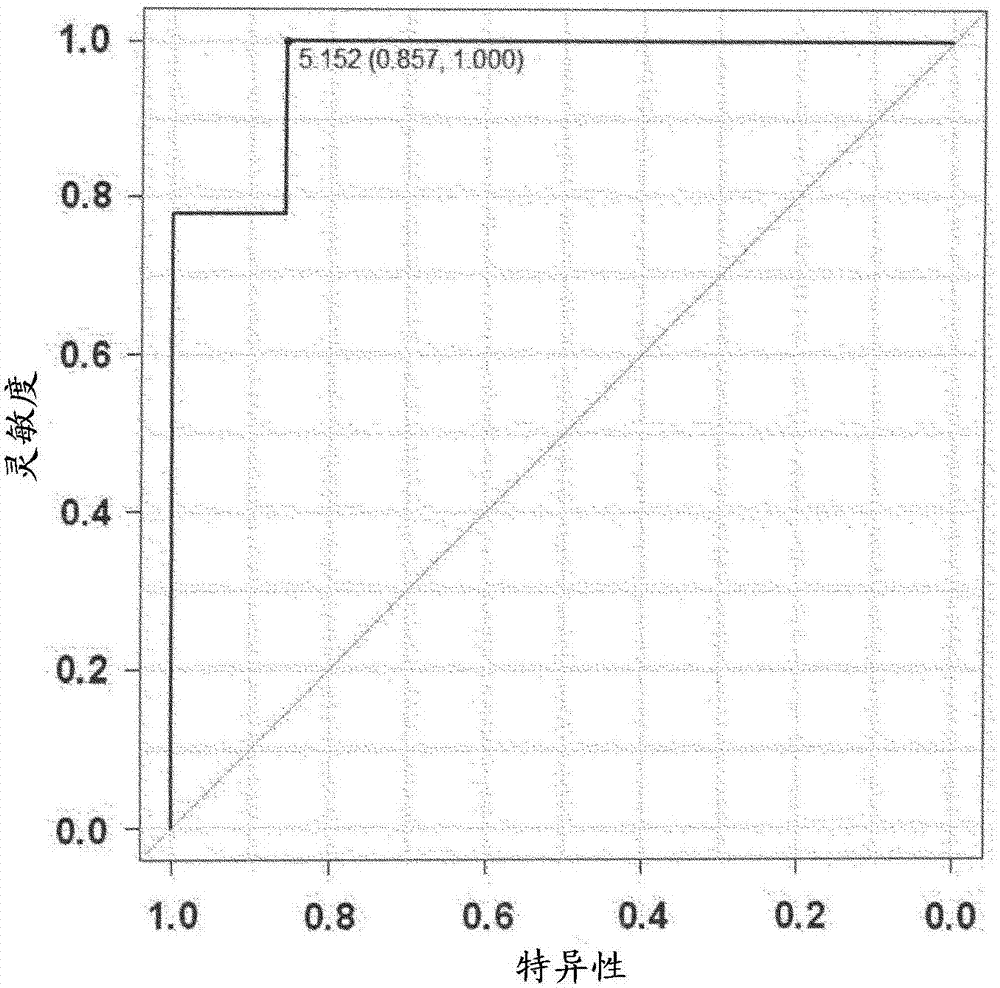
Drainage type kit for early diagnosis of diabetic nephropathy
ActiveCN114152759AAvoid exposureIncrease credibilityDisease diagnosisBiological testingNephrosisEngineering
Owner:XUZHOU MEDICAL UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap