In vitro synthesis of a layered cell sorted tissue
a layered cell and tissue technology, applied in the field of in vitro synthesis of layered cell sorted tissue, can solve the problems of insufficient remedy, inferiority of artificial tissues to their counterparts in vivo, and overgrowth of fibroblasts, and achieve the effect of superior assay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example1
Preparation of Skin Cells
[0049] Briefly, newborn foreskin was trimmed, cut into small pieces, and placed in dispase at 4.degree. C. overnight. The dispase-treated foreskin was transferred into new petri dishes and washed with phosphate buffered saline. Epidermis was physically separated from dermis with forceps and treated with 0.3% trypsin at 37.degree. C. for 30 minutes. The trypsin was then neutralized with soybean trypsin inhibitor (Sigma, St. Louis, Mo.). The detached keratinocytes were collected using a clinical centrifuge. The keratinocyte pellet was resuspended in SFM (Gibco-BRL, Grand Island, N.Y.) media, and plated on collagen coated dishes. After 3-4 days, medium to large colonies of primary keratinocytes were visible (10-20 cells large), and the plates were refed with SFM. After 7 days the plates were then split 1:3 using standard trypsinization methods. The keratinocytes were then cultured for two to three passages in a 37.degree. C. incubator with 5% CO.sub.2 then used in
example 2
Medium Used To Feed Skin Cells
[0051] The medium used to grow the in vitro layered cell sorted skin is also an important variable. The components of the medium compatible with the formation of dermal and epidermal layers is listed below.
[0052] 500 ml DMEM (Gibco-BRL cat. no. 11965-092, Grand Island, N.Y.
[0053] 10 ml penicillin / streptomycin
[0054] Add fetal bovine serum to 10% volume
example 3
Method For Making Layered Cell Sorted Skin In Vitro
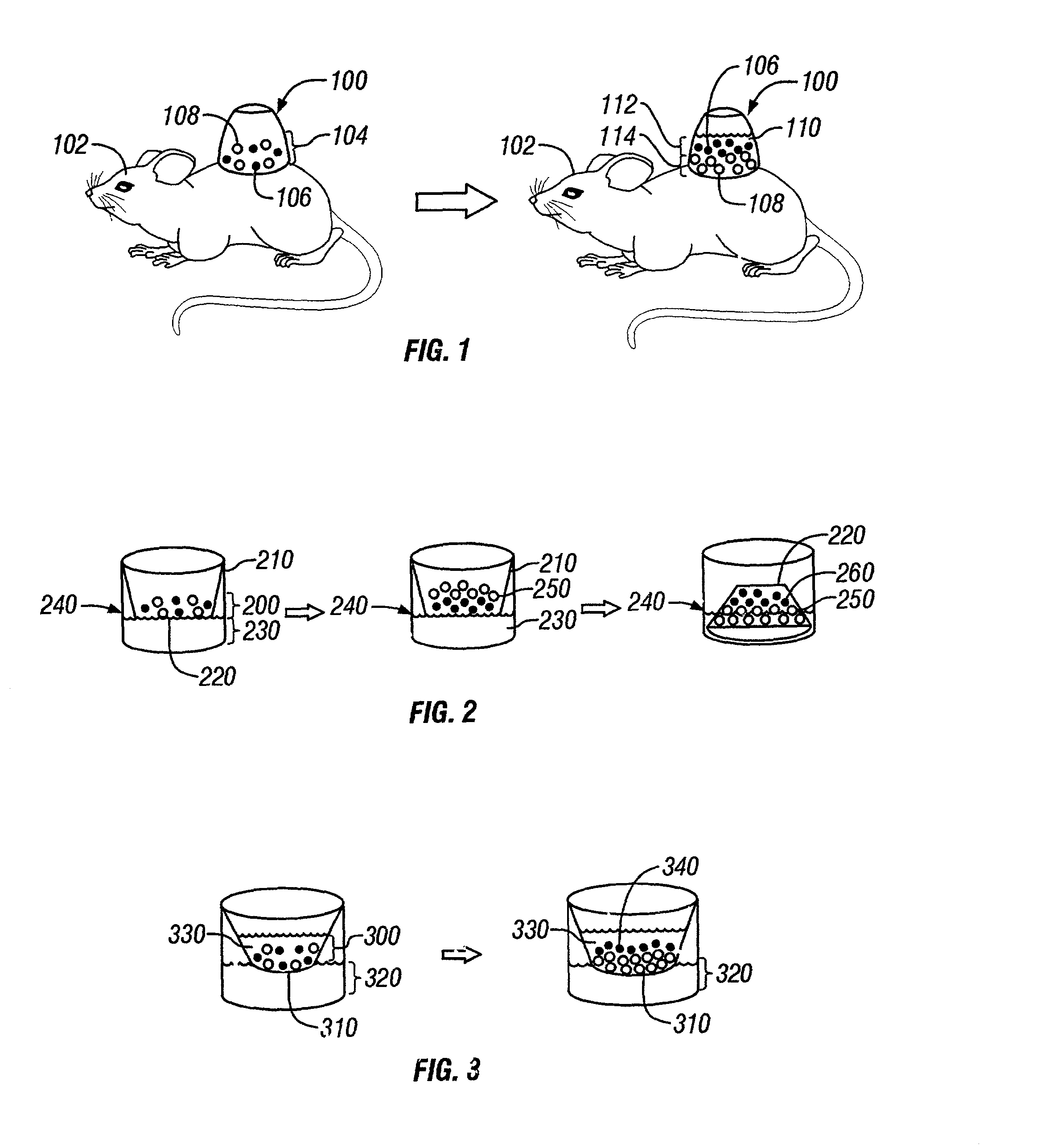
[0055] Transwells were first prepared by coating them with 5 .mu.g / ml fibronectin from human plasma (Sigma, St. Louis, Mo.) and incubating them at 37.degree. C. for 30 minutes. In one centrifuge tube, 4.times.10.sup.6 keratinocytes and 4.times.10.sup.6 fibroblasts were combined, gently mixed, and then centrifuged. Media was then removed without disturbing the cell pellet and the cell pellet incubated on ice for 30 minutes. The cell pellet was then resuspended in an approximately equal volume (50-100 .mu.l) of DMEM with 10% fetal bovine serum (FBS) to create a cell slurry. The resuspended cell slurry was seeded onto the transwell and fed using DMEM with 10% FBS. If the epidermal layer was desired on the membrane, then the culture well and transwell were both fed. However, if epidermis was desired on top, then only the transwell was fed for 24 hours. After that, both wells could be fed. The cells were fed as needed for the first 1 to 3
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap