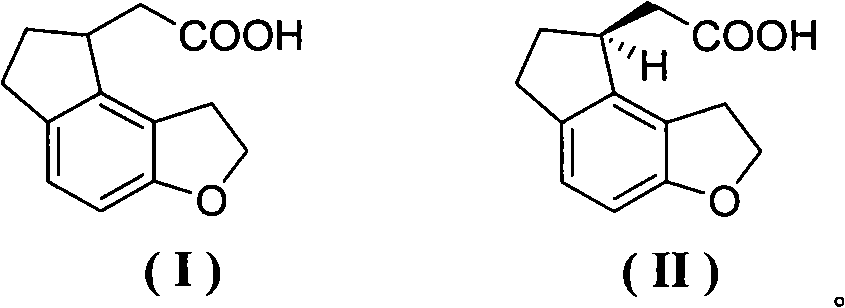
Compound for preparing ramelteon, preparation method thereof and application thereof
A compound and condensation reaction technology, which is applied in the field of preparation of ramelteon, a drug for treating insomnia, can solve the problems of unavailable, high raw material prices, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
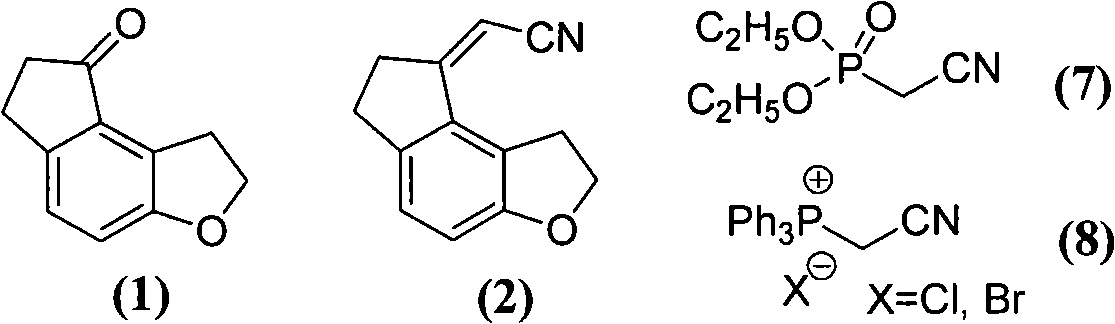
[0060] Preparation of (1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-ylidene)acetonitrile (2)
[0061] Add 21.27 g (0.12 mol) of diethyl cyanomethylene phosphonate, 1,2,6,7-tetrahydro-8H-indeno-[5,4-b]furan-8-one in sequence in the reaction flask 17.42g (0.10mol), 120ml of toluene and 60ml of deionized water, after stirring at room temperature, add 1.62g (0.005mol) of tetrabutylammonium bromide and 20.70g (0.15mol) of anhydrous potassium carbonate, and heat up to 60-70 ℃, keep stirring and react for 8 hours; after the reaction, cool to room temperature, separate the toluene layer, extract the water layer with 30ml of toluene, combine the toluene solution, wash with 25ml of saturated NaCl aqueous solution, evaporate the solvent under reduced pressure, and recrystallize the residue with methanol , to obtain 17.20 g of light yellow crystalline solid, mp: 146-148°C, yield 87.2%.
Embodiment 2
[0063] Preparation of (1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-ylidene)acetonitrile (2)
[0064] Add 40.54 g (0.12 mol) of cyanomethylenetriphenylphosphonium chloride, 1,2,6,7-tetrahydro-8H-indeno-[5,4-b]furan-8- Ketone 17.42g (0.10mol) and toluene 200ml, after stirring at room temperature evenly, put in an ice bath and cool to 0-5°C, add 15% sodium methoxide methanol solution (0.13mol) dropwise, remove the ice bath, and stir at room temperature React for 2.5h, add 100ml of deionized water to the reaction bottle, stir for 30min, pour the reaction solution into a separatory funnel, separate the organic layer, and wash with 40ml of saturated aqueous NaCl solution, evaporate the solvent under reduced pressure, and reconstitute the residue with methanol Crystallized to obtain 16.51 g of light yellow crystalline solid, mp: 145-148°C, yield 83.7%.
Embodiment 3
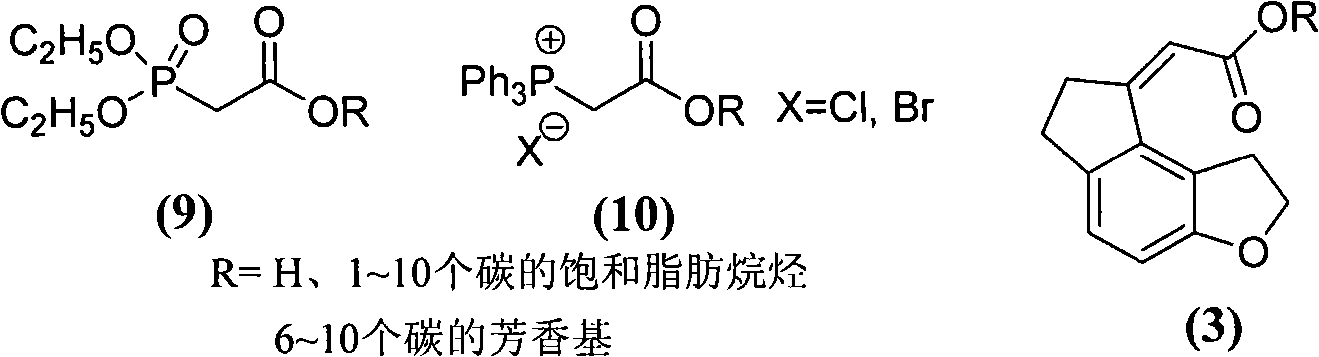
[0066] Preparation of methyl (1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-ylidene)acetate (3a)
[0067] The operation process is the same as in Example 1, except that diethyl cyanomethylene phosphonate is replaced with 2-(diethoxyphosphonic acid group) methyl acetate to obtain (1,6,7,8-tetrahydro-2H-indene And-[5,4-b]furan-8-ylidene)acetate methyl ester as light yellow oily liquid, yield 92.1%. It was directly used in the next reaction without further purification.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap