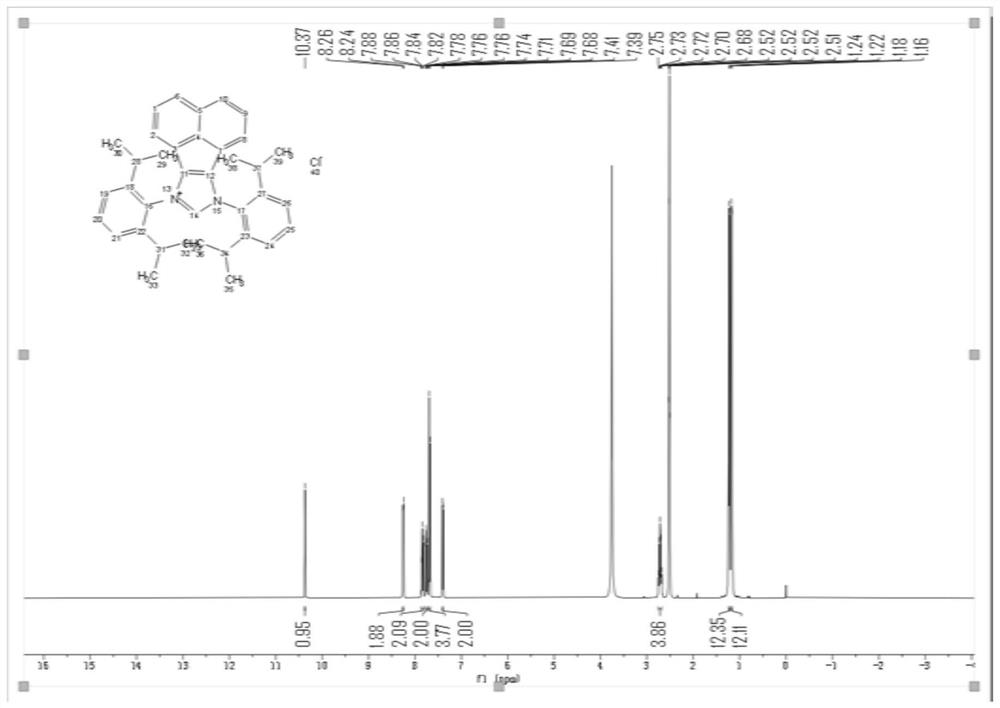
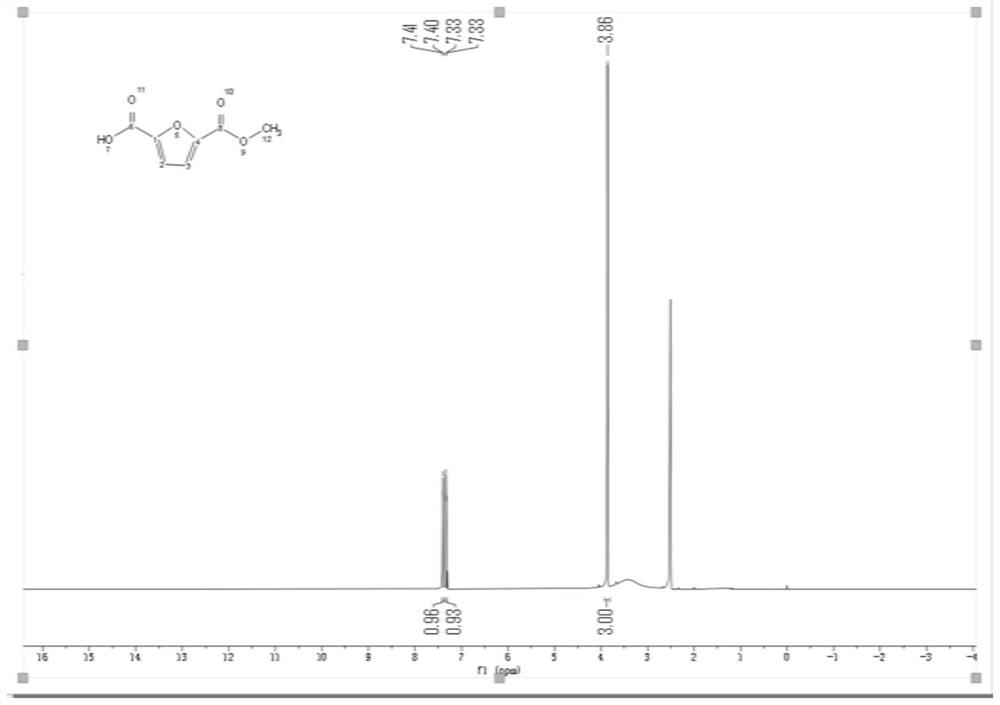
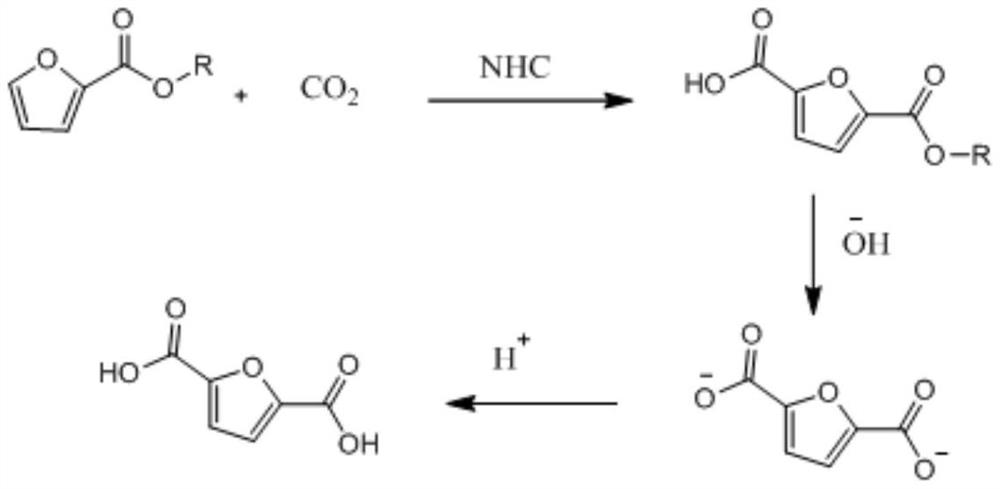
Method for synthesizing 2,5-furandicarboxylic acid by catalyzing carbon dioxide with n-heterocyclic carbene
A technology for catalyzing carbon dioxide and furandicarboxylic acid with carbene, which is applied in organic chemistry and other fields to achieve the effects of high yield, high yield and simple reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1: Add 1.26g of methyl furoate, 0.118g of azacyclic carbene and 44.2mg of rhodium acetate dimer into a dry flask, add 30mL of DMF, and vacuum into CO 2 , and reacted at 150° C. for 48 hours to obtain the crude product of methyl 2,5-furandicarboxylate 2-carboxylate. After column chromatography, 1.47 g of methyl 2,5-furandicarboxylate was obtained with a yield of 86.41%.
[0031] Step 2: 1.47g of 2,5-furandicarboxylic acid methyl 2-carboxylate and sodium hydroxide were dissolved in methanol, and reacted at 60°C for 14 hours to obtain 1.45g of 2,5-furandicarboxylic acid salt crude product with a yield of 100% .
[0032] Step 3: Remove the solvent from 1.45 g of crude 2,5-furandicarboxylate in vacuo, add an appropriate amount of deionized water, and extract three times with an appropriate amount of dichloromethane; acidify the organic layer with concentrated hydrochloric acid until PH = 2; use a heterogeneous solution 250 mL of ethyl acetate for extraction. The organic
Embodiment 2
[0034] Step 1: Add 1.40g of ethyl furoate, 0.118g of azacyclic carbene and 44.2mg of rhodium acetate dimer into a dry flask, add 30mL of DMF, and vacuum into CO 2 , reacted at 150°C for 48h to obtain a crude product of ethyl 2,5-furandicarboxylate 2-carboxylate, and obtained 1.53g ethyl 2,5-furandicarboxylate through column chromatography, with a yield of 83.08%.
[0035] Step 2: Dissolve 1.53g of ethyl 2,5-furandicarboxylate and sodium hydroxide in methanol and react at 60°C for 14 hours to obtain 1.396g of crude 2,5-furandicarboxylate, with a yield of 100% .
[0036] Step 3: 1.396g of crude 2,5-furandicarboxylate was removed from the solvent in vacuo, added an appropriate amount of deionized water, and extracted three times with an appropriate amount of dichloromethane; the organic layer was acidified with concentrated hydrochloric acid until PH = 2; the heterogeneous solution was used 250 mL of ethyl acetate for extraction. The organic layer was dried over anhydrous sodium s
Embodiment 3
[0038] Step 1: Add 1.54g propyl furoate, 0.118g azacyclic carbene and 44.2mg rhodium acetate dimer to a dry flask, add 30mL DMF, and vacuum into CO 2 , and reacted at 150°C for 48 hours to obtain a crude product of 2,5-furandicarboxylic acid 2-propyl carboxylate. After column chromatography, 1.47g of 2,5-furandicarboxylic acid 2-propyl carboxylate was obtained, and the yield was 74.18%.
[0039] Step 2: Dissolve 1.47g of 2,5-furandicarboxylic acid propyl 2-carboxylate and sodium hydroxide in methanol and react at 60°C for 14 hours to obtain 1.247g of 2,5-furandicarboxylate crude product with a yield of 100% .
[0040] Step 3: 1.247g of crude 2,5-furandicarboxylate was vacuum removed from the solvent, added an appropriate amount of deionized water, and extracted three times with an appropriate amount of dichloromethane; the organic layer was acidified with concentrated hydrochloric acid until PH = 2; the heterogeneous solution was used 250 mL of ethyl acetate for extraction. The
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap