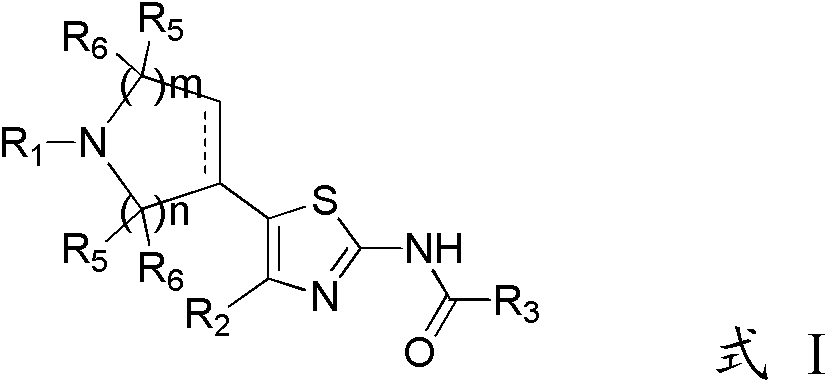
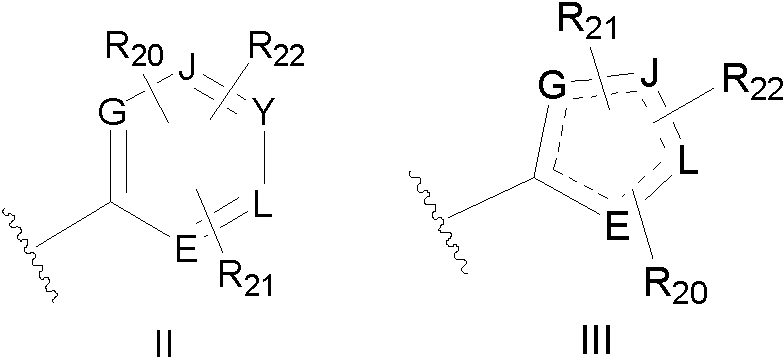
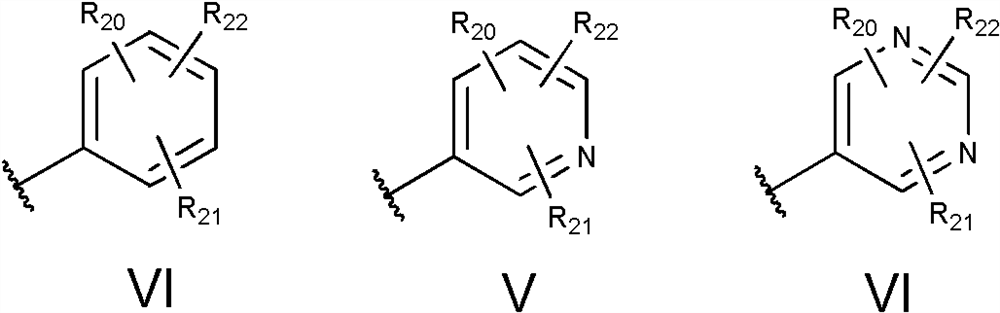
2-amidothiazole derivatives and its preparation method and use
A drug and propyl-based technology, applied in the treatment of diseases mediated by thrombopoietin, can solve serious, limited application, liver toxicity and side effects, etc., achieve excellent metabolic stability, excellent effect of promoting platelet production, and promote platelet production and the effect of megakaryocyte production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] Example 1 1-(3-chloro-5-{[4-(4-chlorothien-2-yl)-5-(1-cyclohexyl-1,2,3,6-tetrahydropyridin-4-yl )thiazol-2-yl]carbamoyl}pyridin-2-yl)piperidine-4-carboxylic acid (compound L1)
[0222]
[0223] Step 1: Synthesis of 4-[2-amino-4-(4-chlorothiophen-2-yl)thiazol-5-yl]-3,6-dihydropyridine-1(2H)-carboxylic acid tert-butyl ester
[0224] 5-Bromo-4-(4-chlorothiophen-2-yl)-2-amino-thiazole (900mg, 3.1mmol), N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4 - pinacol borate (960mg, 3.1mmol), anhydrous sodium carbonate (920mg, 7.3mmol) and tetrakistriphenylphosphine palladium (180mg, 0.2mmol), dissolved in a mixed solvent of ethylene glycol dimethyl ether and water Medium (45mL, V:V=2:1). Reacted at 70°C for 1.5h under the protection of nitrogen. Cool down to room temperature, filter, and concentrate the filtrate to obtain a crude product, which is purified by silica gel column chromatography to obtain 1.1 g of the title compound.
[0225] ESI-MS(m / z): 398.2[M+H] +
[022
Embodiment 2
[0242] Example 2 1-(3-chloro-5-{[4-(4-chlorothien-2-yl)-5-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl )thiazol-2-yl]carbamoyl}pyridin-2-yl)piperidine-4-carboxylic acid (compound L2)
[0243]
[0244] Using a method similar to Example 1, but in the first step, replace N- Starting from tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester (960 mg, 3.1 mmol), the title was prepared by substituting paraformaldehyde for cyclohexanone in the fifth step Trifluoroacetate salt of compound, 32 mg.
[0245] 1 H NMR (400MHz, DMSO-d 6 )δ12.73(s, 1H), 12.31(s, 1H), 9.26(s, 1H), 8.84(d, J=2.1Hz, 1H), 8.40(d, J=2.1Hz, 1H), 7.62( d, J=1.5Hz, 1H), 7.52(d, J=1.5Hz, 1H), 6.29(s, 1H), 4.00-3.97(d, J=13.1Hz, 2H), 3.68(s, 1H), 3.60-3.52(m, 1H), 3.46(d, J=11.6Hz, 2H), 3.07-3.01(m, 2H), 2.93(d, J=12.1Hz, 2H), 2.82(d, J=3.9Hz , 3H), 2.56-2.54 (m, 1H), 1.95 (d, J=10.5Hz, 2H), 1.72-1.63 (m, 2H), 1.38 (s, 3H), 1.31-1.18 (m, 2H).
[0246] ESI-MS(m / z): 578.2[M+H] + .
Embodiment 3
[0247] Example 3 1-(3-chloro-5-{[4-(4-chlorothien-2-yl)-5-(1-cyclohexyl-1,2,5,6-tetrahydropyridin-3-yl )thiazol-2-yl]carbamoyl}pyridin-2-yl)piperidine-4-carboxylic acid (compound L3)
[0248]
[0249] Using a method similar to Example 1, but in the first step, replace N- 32 mg of the trifluoroacetic acid salt of the title compound were prepared from tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester (960 mg, 3.1 mmol).
[0250] 1 H NMR (400MHz, DMSO-d 6 )δ12.89(s, 1H), 12.26(s, 1H), 9.80(s, 1H), 8.85(d, J=2.1Hz, 1H), 8.42(d, J=2.1Hz, 1H), 7.63( s, 1H), 7.42(s, 1H), 6.30(s, 1H), 4.00-3.97(m, 3H), 3.82(d, J=15.4Hz, 1H), 3.62(s, 1H), 3.29-3.24 (m, 1H), 3.07-3.01(t, J=11.9Hz, 2H), 2.93(d, J=12.1Hz, 2H), 2.69-2.53(m, 2H), 2.09-1.93(m, 4H), 1.82 (d, J=10.4Hz, 2H), 1.71-1.59 (m, 3H), 1.48-1.39 (m, 2H), 1.32-1.23 (s, 3H), 1.14-1.05 (m, 2H).
[0251] ESI-MS(m / z): 646.2[M+H] + .
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap