Method for determining iodine value of palm oil
A measurement method, palm oil technology, applied in the direction of material thermal development, etc., can solve the problems of palm oil iodine value determination operation complexity, reagent toxicity, etc., to achieve accurate and reliable measurement results, safe and simple operation, and remove the effect of human interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Establishment of a standard control curve between the iodine value of a palm oil sample and the crystallization exothermic peak.
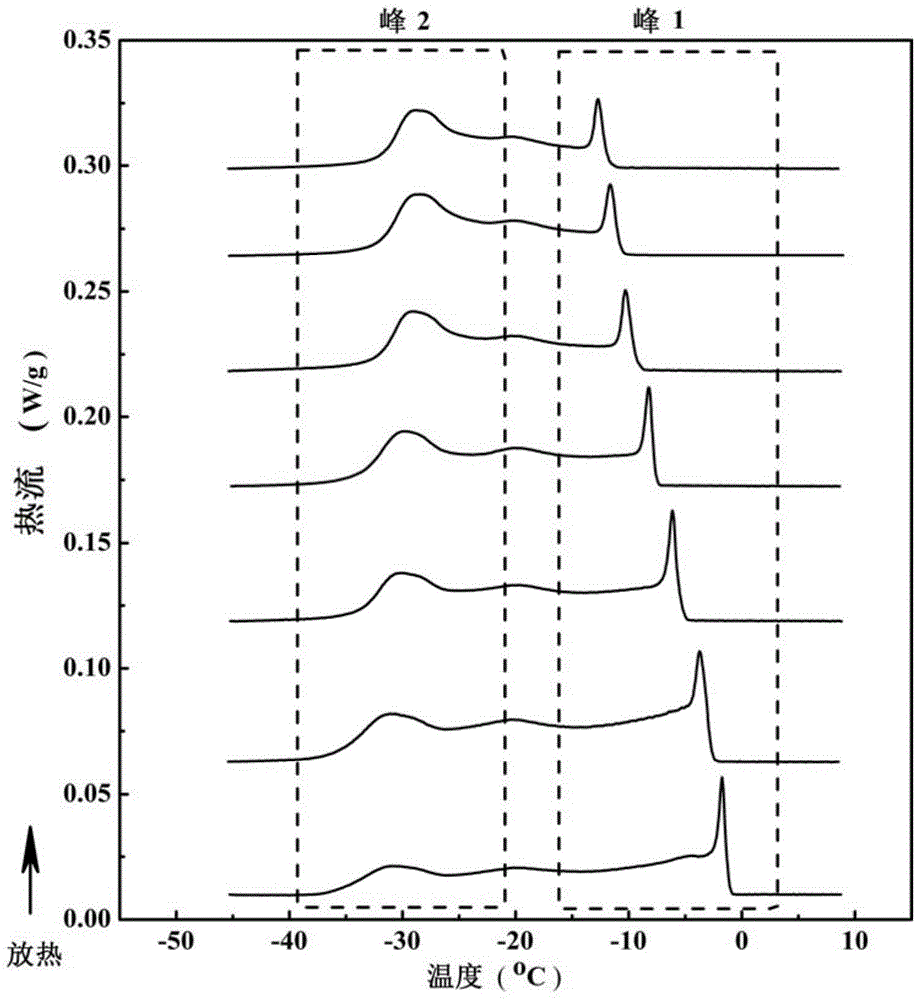
[0043] Take 30 different grades of palm oil products with known iodine value of 65-75, heat these samples at 70°C under stirring, dissolve them all, and keep the temperature for 30 minutes; take 5 mg of palm oil samples to differential In the scanning calorimeter crucible, seal it with a matching crucible lid, and put it into the differential scanning calorimeter. The crucible was heated up to 70°C at 5°C / min, kept at a constant temperature for 30 minutes, and then cooled to -45°C at 5°C / min to measure the heat change during palm oil crystallization. The obtained exotherm spectra of different samples are as follows: figure 1 shown.
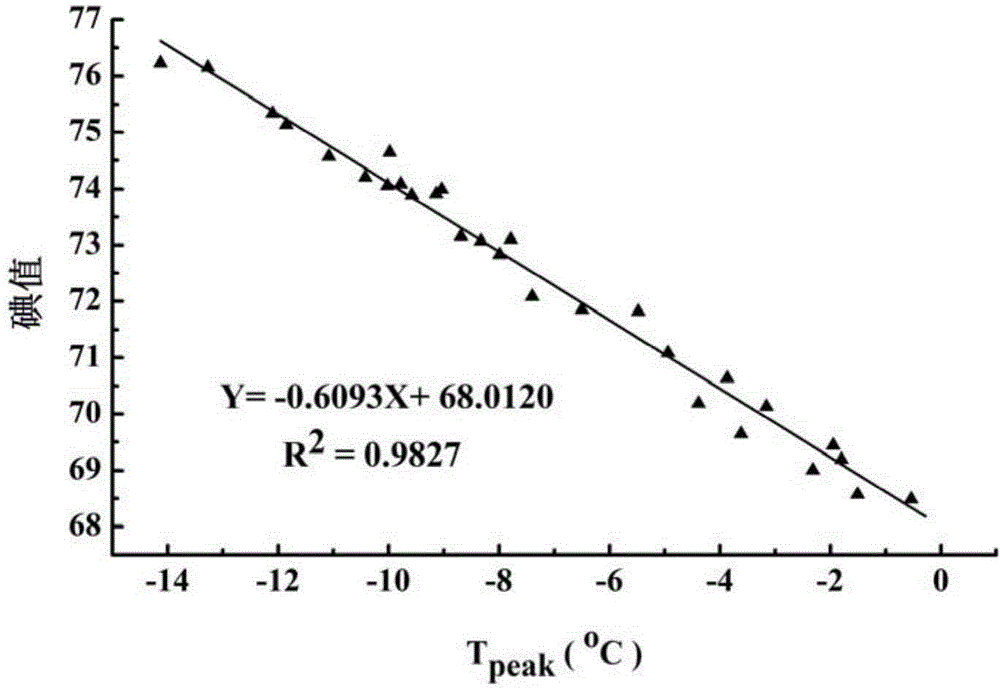
[0044] The iodine value of the above-mentioned oil sample is respectively different from the oil crystallization peak 1 measured by differential scanning calorimetry (the crystallization point temp
Embodiment 2
[0045] Example 2: Using differential scanning calorimetry T on - Iodine value standard control curve to determine the iodine value of palm oil.
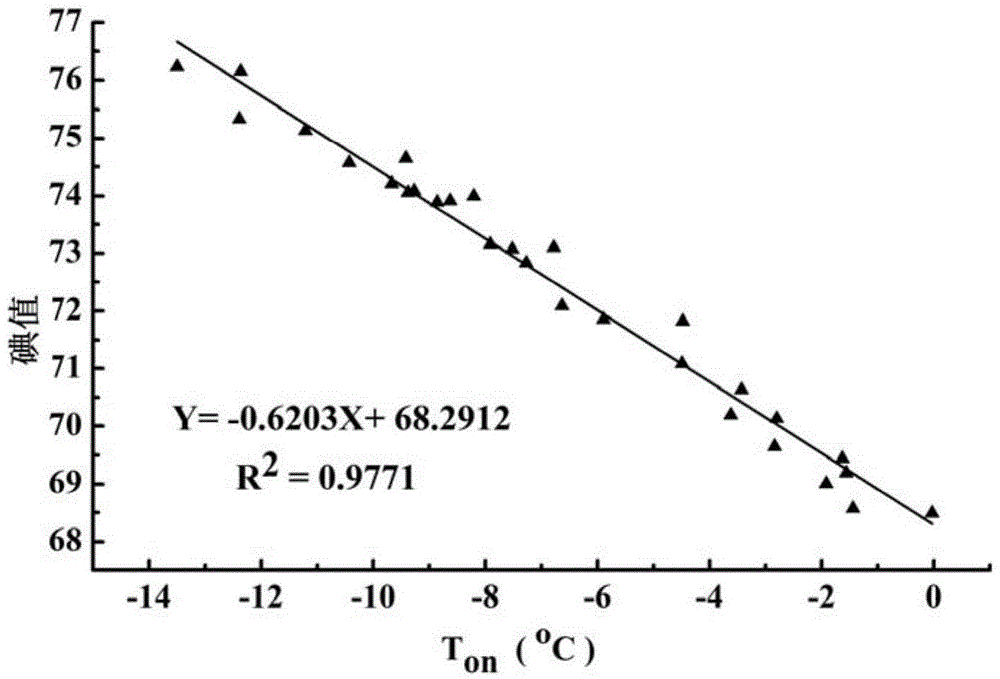
[0046] The iodine value of the palm oil sample to be analyzed was determined by differential scanning calorimetry. First, 10 mg of the palm oil sample was added to the crucible of the differential scanning calorimeter, sealed with a matching crucible cover, and put into the differential scanning calorimeter. The crucible was heated up to 70°C at 5°C / min, kept at a constant temperature for 30 minutes, and then cooled to -45°C at 5°C / min to measure the heat change during palm oil crystallization. By this method, measure the crystallization point temperature T of the crystallization peak 1 of this sample on is -12.03°C, compare this value with the fitted T on - iodine value standard curve (results such as figure 2 Shown) is compared and utilizes the correlation formula Y=-0.6203X+68.2912 in the figure to calculate and obtain the io
Embodiment 3
[0047] Example 3: Using differential scanning calorimetry T on - Iodine value standard control curve to determine the iodine value of palm oil.
[0048] The iodine value of the palm oil sample to be analyzed was determined by differential scanning calorimetry. First, 5 mg of the palm oil sample was added to the crucible of the differential scanning calorimeter, sealed with a matching crucible cover, and put into the differential scanning calorimeter. The crucible was heated to 60°C at 5°C / min, kept at a constant temperature for 40min, and then cooled to -50°C at 5°C / min to measure the heat change during palm oil crystallization. By this method, measure the crystallization point temperature T of the crystallization peak 1 of this sample on is -10.03℃, and this value is compared with the fitted T on - iodine value standard curve (results such as figure 2 Shown) is compared and utilizes the association formula Y=-0.6203X+68.2912 in the figure to calculate and obtain the iodi
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap