Preparation method of varenicline tartrate intermediate
A technology of varenicline tartrate and intermediates, applied in the direction of organic chemistry and the like, can solve problems such as being unsuitable for large-scale production and reducing yield, and achieve the effects of being conducive to large-scale production, mild and safe method, and good yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
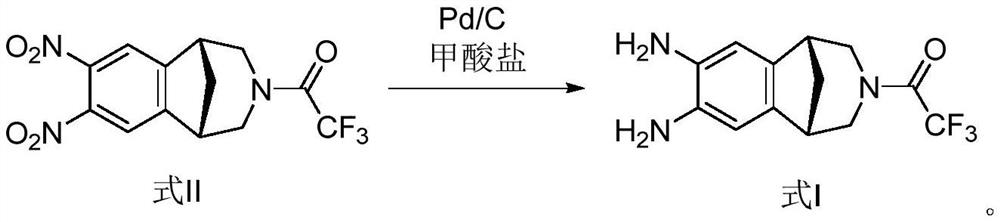
[0037] Ammonium formate solution: Add 4.4 g of ammonium formate into 9 ml of purified water and stir to dissolve.
[0038] Add 36ml of methanol to the reaction flask. Under the protection of nitrogen, 10% Pd / C 0.5g (dry basis) and the compound of formula II (3g, 8.7mmol) were added successively, and stirred for 5-10 minutes.
[0039] Ammonium formate solution was added dropwise. After dropping, stir at 30°C to 40°C for 40 to 60 minutes.
[0040] TLC detection, after the reaction is complete, add 20ml of dichloromethane to the reaction solution, spread diatomaceous earth to filter, add 30ml of purified water to the filtrate, leave to stand for layering after stirring, extract the aqueous phase twice with dichloromethane, combine the organic phase, dried with 10 g of anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 2.25 g, yield: 90.8%, purity: 98.3%, and was directly used for feeding in the next step.
Embodiment 2
[0042] Ammonium formate solution: Add 4.4 g of ammonium formate into 9 ml of purified water and stir to dissolve.
[0043] Add 36ml of ethanol to the reaction flask. Under the protection of nitrogen, 10% Pd / C 0.5g (dry basis) and the compound of formula II (3g, 8.7mmol) were added successively, and stirred for 5-10 minutes.
[0044] Ammonium formate solution was added dropwise. After dropping, stir at 30°C to 40°C for 40 to 60 minutes.
[0045] TLC detection, after the reaction is complete, add 20ml of dichloromethane to the reaction solution, spread diatomaceous earth to filter, add 30ml of purified water to the filtrate, leave to stand for layering after stirring, extract the aqueous phase twice with dichloromethane, combine the organic phase, dried with 10 g of anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 2.23 g, yield: 90.0%, purity: 98.2%, and was directly used for feeding in the next step.
Embodiment 3
[0047] Ammonium formate solution: Add 4.4 g of ammonium formate into 9 ml of purified water and stir to dissolve.
[0048] Add 36 ml of N-methylpyrrolidone into the reaction flask. Under the protection of nitrogen, 10% Pd / C 0.5g (dry basis) and the compound of formula II (3g, 8.7mmol) were added successively, and stirred for 5-10 minutes.
[0049] Ammonium formate solution was added dropwise. After dropping, stir at 30°C to 40°C for 40 to 60 minutes.
[0050]TLC detection, after the reaction is complete, add 20ml of dichloromethane to the reaction solution, spread diatomaceous earth to filter, add 30ml of purified water to the filtrate, leave to stand for layering after stirring, extract the aqueous phase twice with dichloromethane, combine the organic phase, dried with 10 g of anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 2.16 g, yield: 87.1%, purity: 98.6%, and was directly used for feeding in the next step
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap