Animal model of neuronal injury
a neuronal injury and animal model technology, applied in the field of animal models of neuronal injury, can solve the problems of inability to perform rapid pharmacodynamics studies, and achieve the effect of easy creation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Induction of Cognitive Decline and Decreased Memory Recall by Chronic lntracerebroventricular infusion (ICV) of Fibrinogen to the CNS of a Mammal
[0107]Fibrinogen was delivered to the central nervous system (CNS) of wild-type mice by ICY. Briefly, fibrinogen (5 mg / ml) or artificial cerebrospinal fluid (ACSF), a control, was infused into the CNS of the mice using 14-day Alzet pumps through a cannula implanted in the right lateral ventricle. The concentration of the fibrinogen solution was selected based on its physiological range in the plasma. Five days after pump implantation, the mice were trained in the Morris water maze and tested after removal of the target platform 24 hours after the last learning trial. Fibrinogen-infused mice had impaired memory recall, as exhibited by no difference in the number of crossings of the target platform (FIG. 1A) or time spent in the target quadrant (FIG. 1B) The ACSF-infused control mice crossed the target platform significantly more times and als
example 2
Induction of Neuronal Damage and Cognitive Decline by Injection of Fibrinogen in the Dentate Gyrus of a Mammalian Brain
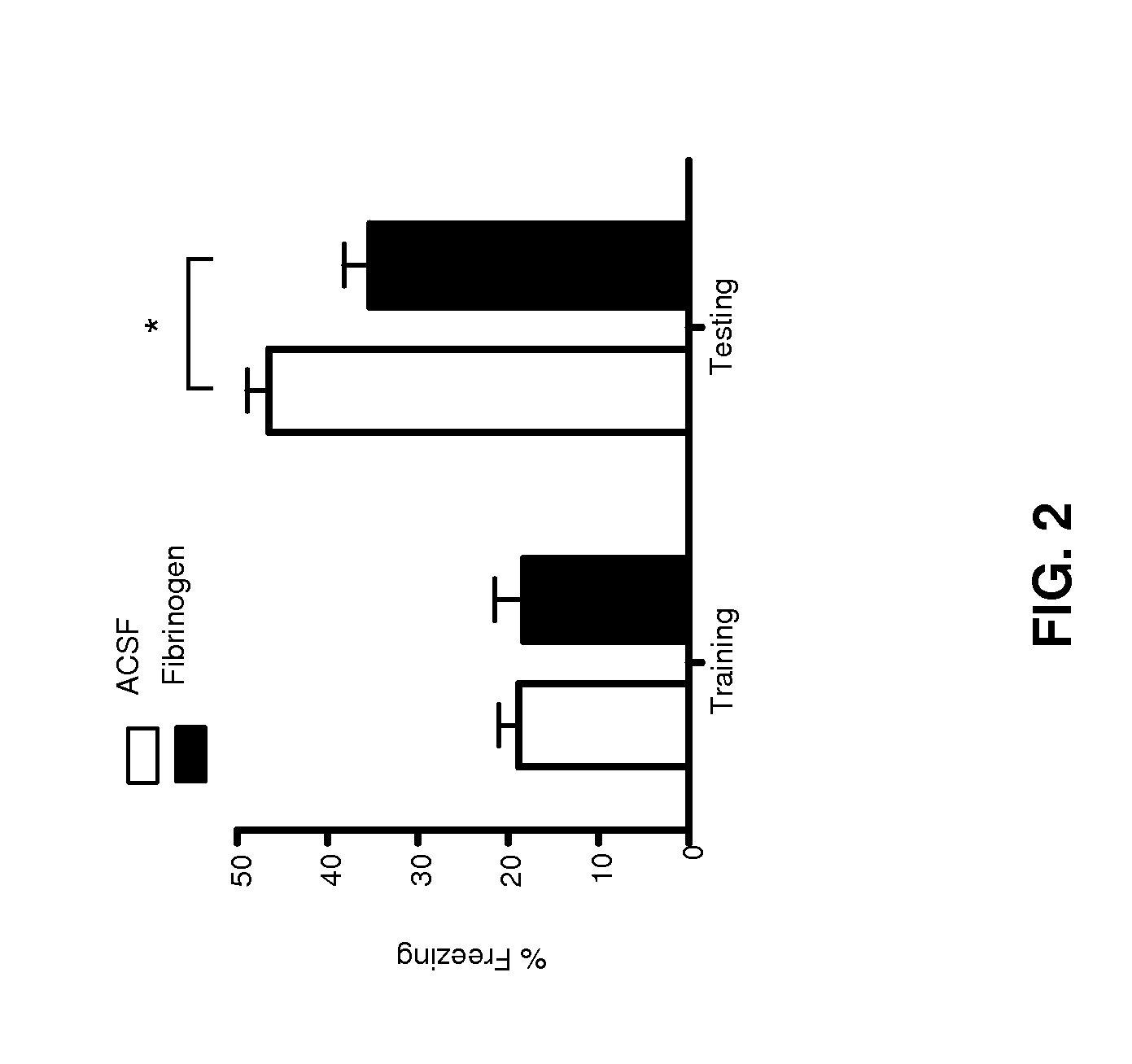
[0109]To assess the effect of an increase in the local concentration of fibrinogen in a specific region of interest in the brain, mice received a single stereotactic injection of either 5 μg fibrinogen (1 μl of 5 mg / ml fibrinogen) or 1 μl control (ACSF) in the dentate gyrus. The effects on memory retrieval were assessed in the contextual fear conditioning test (Riley C et al., (2012) Expert review of neurotherapeutics 12(3):323-333.). Mice were placed in a novel environment and given a brief foot shock (training day; day 6 post injection). Memory was assessed by placing the mice in the same environment again one day after the training day (testing day, day 7 post-injection) and freezing (anxiety) behavior was recorded. Percentage of freezing is a direct measure of memory retrieval. Fibrinogen injected animals presented a significant decrease in percentage of freezing
example 3
Reduction of Dendritic Spine Density by Injection of Fibrinogen in the Cortex of a Mammalian Brain
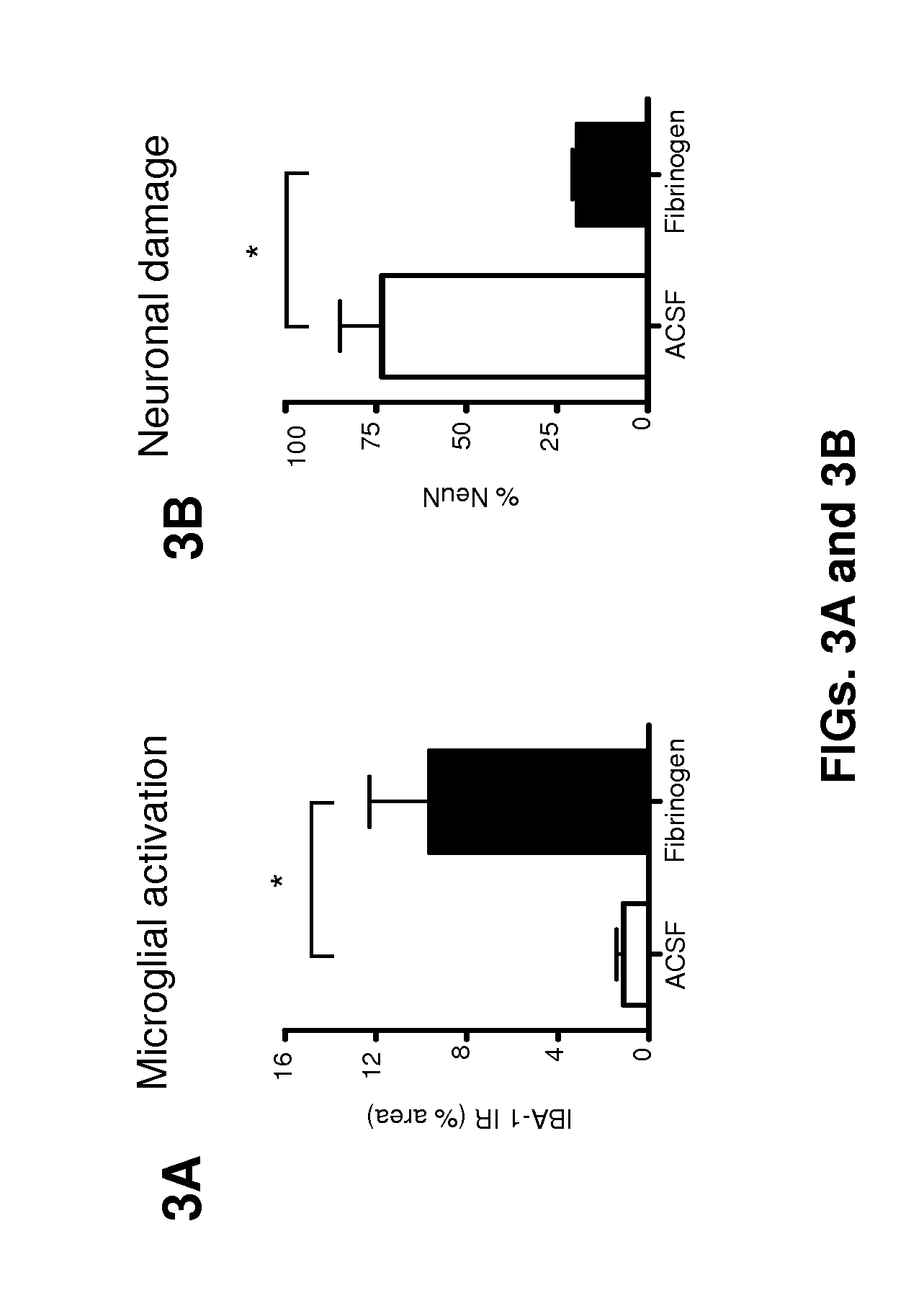
[0112]Fibrinogen injection in the cortex reduces dendritic spine density. Dendritic spines are the major site of excitatory synaptic inputs in the brain (Nimchinsky EA, et al., Annu Rev Physiol. 64:313-353). Novel spine formation and pre-existing spine elimination reflect the structural consequences of dynamic changes in neuronal activity at the cellular level (Grutzendler J & Gan WB (2006) NeuroRx 3(4):489-496; Trachtenberg J T et al., (2002) Nature 420(6917):788-794; Grutzendler J et al. (2002) Nature 420(6917):812-816.). Thus, dynamic changes in spine numbers and morphology are considered the cellular mechanisms underlying memory and learning (Holtmaat Aet al. (2006) Nature 441 (7096):979-983). To assess the effect of a local concentration of fibrinogen on spine morphology, we injected fibrinogen by stereotactic injection into the cortex, and the brains were examined 3 days later by Gol
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap