Oral Antagonist Compositions For Nicotine Burning Relief
a technology of oral antagonists and compositions, applied in the field of analgesic compositions, can solve the problems of less efficient reduction of nicotine irritation, not optimized for targets, and many short-term disadvantages of oral nicotine intake, so as to and reduce the peak perceived nicotine irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0334]Oral Analgesic Compositions with or without Nicotine
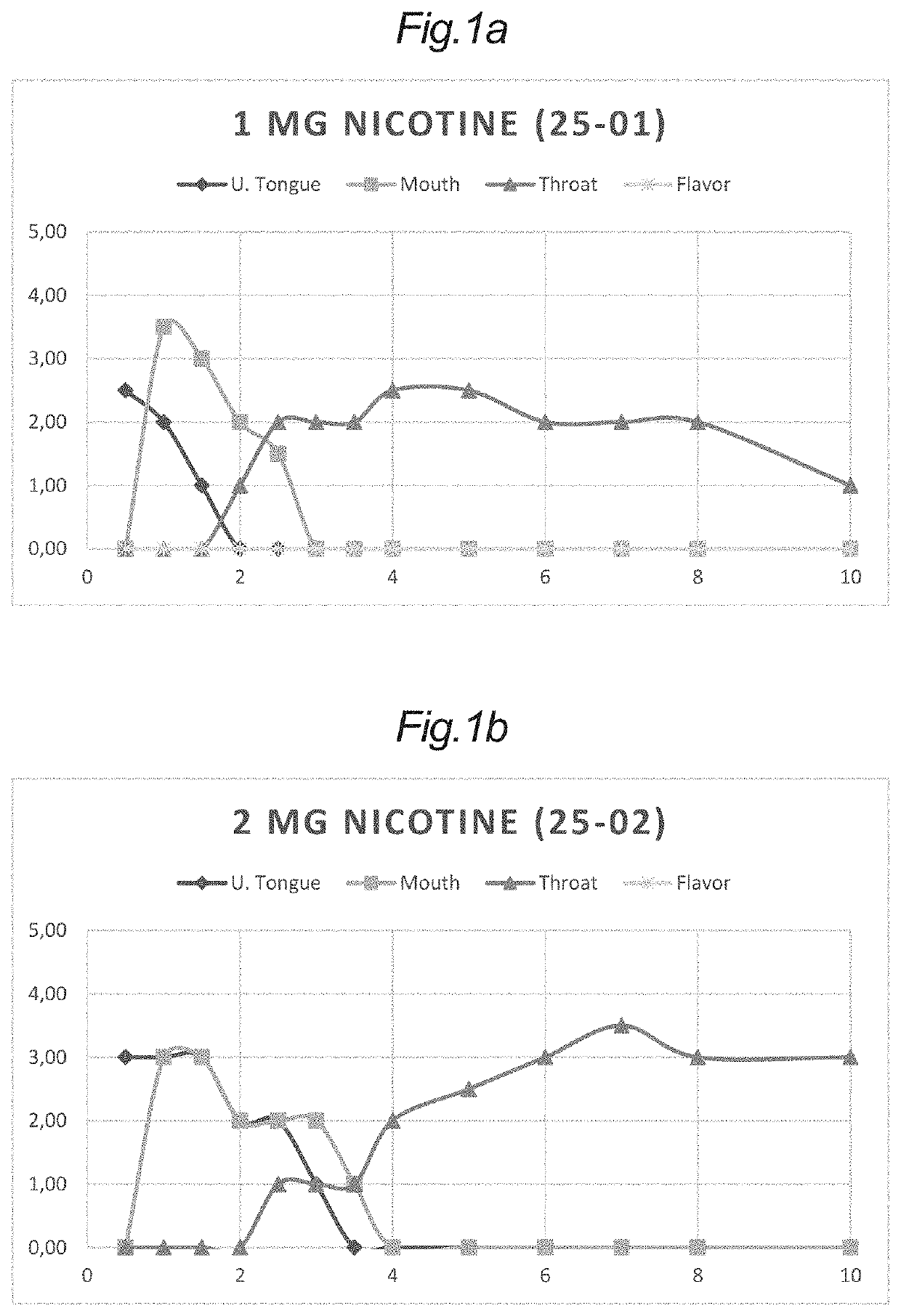
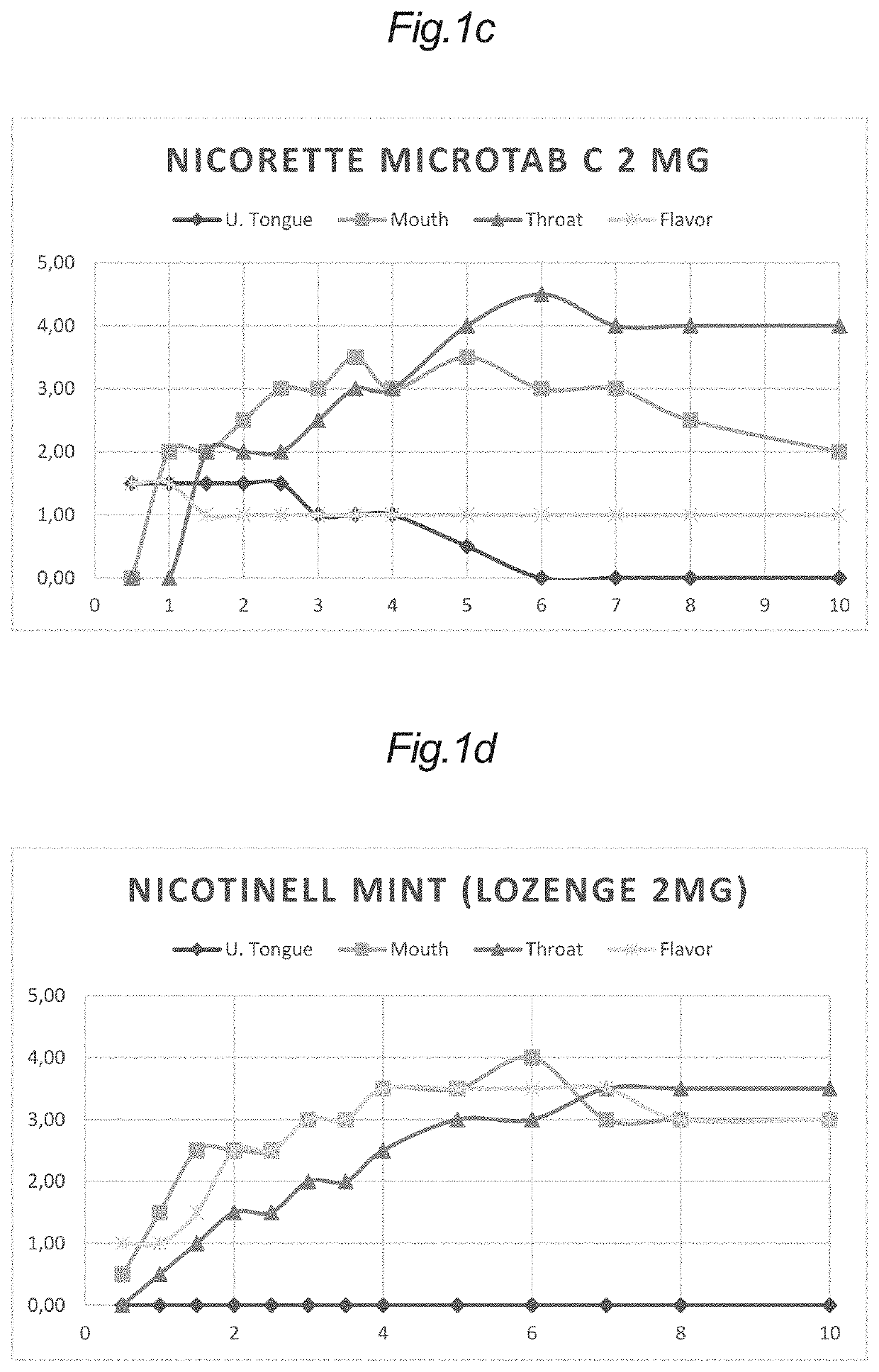
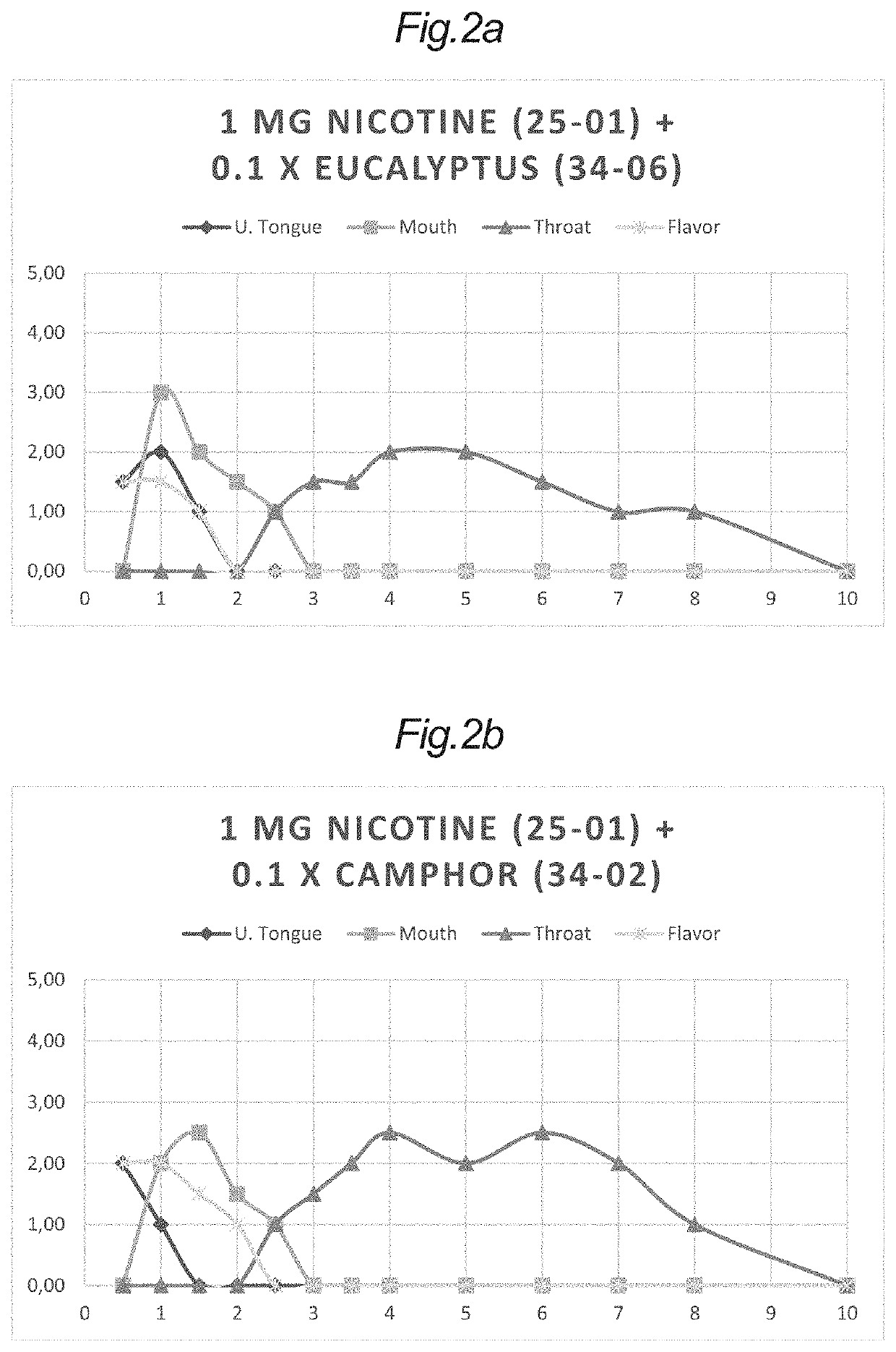
TABLE 1Oral analgesic compositions. Amounts of raw materials are given in mg.(25-01)(25-02)(34-06)(34-04)(34-02)(25-07)Nicotine API*3.096.18————Na2CO35.010.0————Eucalyptol——10.0———Oleic Acid———10.0——Camphor————2.0—WS-12—————2.0Mannitol76.9168.8271.071.085.587.0Crospovidone8.08.07.57.57.57.5Syloid——10.010.00.5—Micro-5.05.0———2.5crystallinecellulose (MCC)Magnesium2.02.0————StearateSodium Stearyl——1.51.54.51.0FumarateTotal100.0100.0100.0100.0100.0100.0*The nicotine API used contains 32.4% nicotine implying that a formulation with 3.088 mg API contains 1 mg of nicotine
[0335]Examples of specific raw materials used in the table are as follows:
[0336]Nicotine may eg be nicotine bitartrate dihydrate (NBT)
[0337]Eucalyptol may eg be Eucalyptol Nat available from Symrise
[0338]Oleic acid may eg be NF-LQ-(MH) available from Croda Inc
[0339]Camphor may eg be Camphor USP available from Rochem International Inc
[0340]WS-12 may eg be Symcool WS-12
example 2
[0345]Fast Dissolving Tablets (FDT) with or without Nicotine
[0346]Fast disintegrating tablets (FDT) were prepared based on the compositions of Example 1. The tablets were prepared as follows:
[0347]Raw materials are weighed from bags or buckets into separate weighing containers. For formulations using Syloid, a premix is made by mixing Eucalyptus and Syloid XDP 3050, Camphor and Syloid 244 FP or Oleic acid and Syloid 244 FP respectively.
[0348]All excipients are sifted through an 800-micron sieve into a stainless steel or plastic bin in the following order:[0349]Half the filler / bulk sweetener[0350]The nicotine (if included) and all other excipients including premix, except magnesium stearate and sodium stearyl fumarate[0351]The remaining half of the filler / bulk sweetener
[0352]These are mixed in a Turbula mixer for 4-10 minutes at 25 RPM. Then lubricant, eg. magnesium stearate is sifted through an 800-micron sieve into the mixing bin, and the lubrication is conducted by additional mix
example 3
[0356]Preparation of Samples for Testing
[0357]Samples were prepared based on the tablets from Example 2.
[0358]Tablets with a content of nicotine (25-01) and (25-02) were evaluated in a sensory panel and used as standards for nicotine irritation under the tongue, in the oral cavity and in the throat. These samples were applied in test conditions in a weight of 100 mg, corresponding to the weight of the tablets of Example 2.
[0359]Additionally, tablets without a content of nicotine (34-06), (34-04), (34-02) and (25-07) were combined with the nicotine tablets (25-01) and (25-02) in various configurations.
[0360]In some configurations, one of each of these tablets without nicotine was combined with one of the nicotine tablets to give samples of a nicotine tablet and a single antagonist tablet for evaluation in a sensory panel for nicotine irritation under the tongue, in the oral cavity and in the throat.
[0361]In some other configurations, two of each of these tablets without nicotine were co
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap