A New Contrast Agent of Triiodobenzene Compounds
The technology of a compound and a contrast agent is applied in the field of triiodobenzene compound and a contrast agent containing the compound, 5--N, and can solve the problems of high viscosity, toxicity and hypertonicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
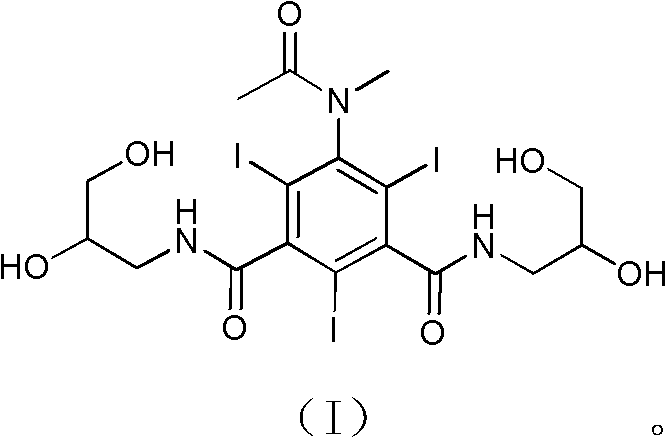
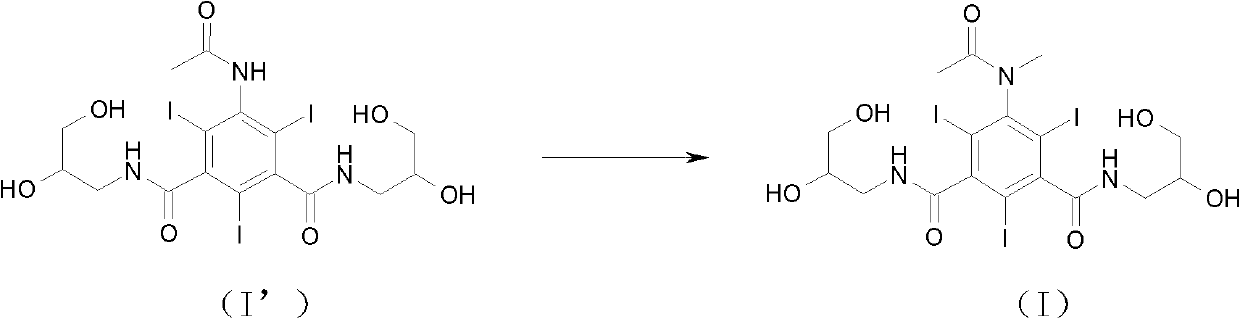
[0026] Example 15-(N-methylacetylamino)-N, N'-bis(2,3-dihydroxy-n-propyl)-2,4,6-triiodoisophthalamide (I)
[0027]
[0028] Under ice-water bath cooling and mechanical stirring, 5-(acetylamino)-N,N'-bis(2,3-dihydroxy-n-propyl)-2,4,6-triiodoisophthalamide (I) (2000g , 2.677mol) (produced by Zhejiang Stellite Pharmaceutical Co., Ltd.), sodium hydroxide (139.2g, 3.480mol) was dissolved in 50% ethylene glycol monomethyl ether aqueous solution, stirred at room temperature, added methyl iodide (380.1g, 2.677mol), TLC followed the reaction process, and the reaction was completed in 6h. Concentrate under reduced pressure and use resin to purify and separate. The specific method is: prepare 5 liters of macroporous adsorption resin regenerated with 40% methanol, pack it into a glass column with a diameter of 100mm, pour the product aqueous solution into it, and let it absorb statically. For more than two hours, gradient elution was carried out with different concentrations of methanol
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap