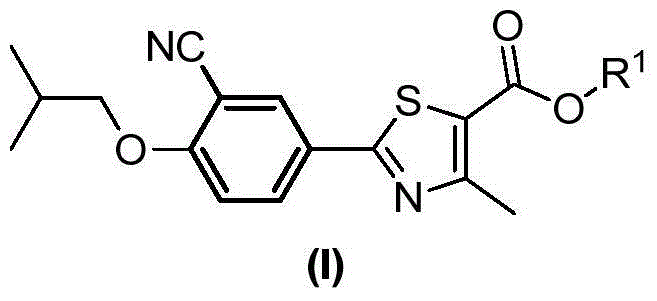
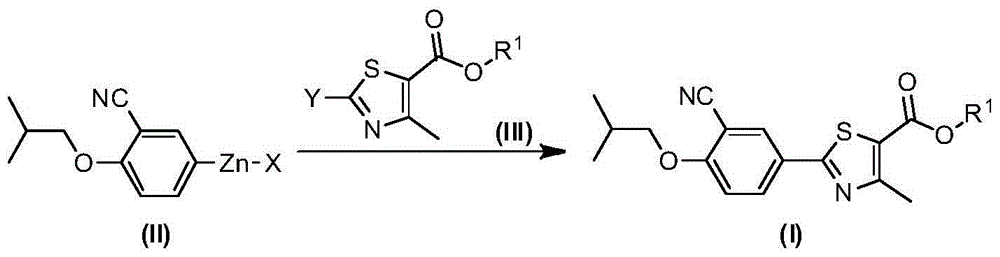
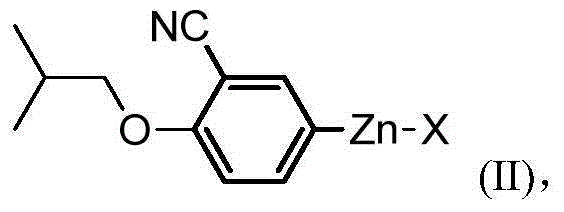
Preparation method of 2-arylnitrile-thiazole derivative
An aryl nitrile thiazole and derivative technology, applied in the direction of zinc organic compounds, organic chemistry, etc., can solve the problems of high cost, unsuitable for industrial amplification, pollution, etc., and achieve the effects of mild conditions, short steps and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0083] Reference Example 1 5-iodo-2-isobutoxybenzonitrile
[0084] 2-Methyl-1-propanol (1.11g, 15.1mmol) was dissolved in N,N-dimethylformamide (20mL), cooled to 0°C, and sodium hydride (0.6g, 15.1mmol, 60% dispersed in mineral oil). After the mixture was stirred at 0°C for 30 minutes, 2-fluoro-5-iodobenzonitrile (2.5 g, 10.1 mmol) was added thereto, returned to room temperature, and stirred overnight. After the reaction was completed, it was quenched with water (30 mL), and extracted with ethyl acetate (100 mL x 3). The combined organic phases were dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography (petroleum ether:ethyl acetate (v / v)=40:1) to obtain the title compound as a yellow liquid (2.7g, 90%) .
[0085] 1 H NMR (400MHz, CDCl 3 )δ(ppm):1.05(d,6H,J=6.7Hz),2.12-2.19(m,1H),3.80(d,2H,J=6.5Hz),6.72(d,1H,J=8.9Hz) ,7.77(dd,1H,J=2.2,8.9Hz),7.80(d,1H,J=2.2Hz).
Embodiment 1
[0086] Example 1 2-zinc bromide-4-methylthiazole-5-ethyl carboxylate
[0087] Under anhydrous and oxygen-free conditions, zinc powder (325mg, 5mmol) and 1,2-dibromoethane (94mg, 0.5mmol) were mixed, and N,N-dimethylformamide (10mL) was added thereto . After the mixture was stirred at reflux for 0.5 hours, it was cooled to room temperature, and trimethylchlorosilane (65 mg, 0.6 mmol) and 5-iodo-2-isobutoxybenzonitrile (602 mg, 2 mmol) were sequentially added thereto. The reaction solution was heated up to 85°C, stirred for 7 hours, cooled, and used directly for the next reaction.
Embodiment 2
[0088] Example 2 Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate
[0089]Under anhydrous and oxygen-free conditions, the above-prepared mixture was added to ethyl 2-bromo-4-methylthiazole-5-carboxylate (249mg, 1mmol) and Pd(PPh 3 ) 4 (116mg, 0.1mmol) in the mixture. Stir at room temperature and monitor the reaction by TLC. After the reaction, brine (20 mL) was added, and extracted with ethyl acetate (100 mL x 3). The combined organic phases were dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography (petroleum ether:ethyl acetate (v / v)=10:1) to give the title compound as a white solid (0.21g, 61%) .
[0090] 1 H NMR (400MHz, CDCl 3 )δ(ppm):1.09(d,6H,J=6.7Hz),1.39(t,3H,J=7.2Hz),2.17-2.24(m,1H),2.76(s,3H),3.90(d, 2H,J=6.5Hz),4.35(q,2H,J=7.2Hz),7.00(d,1H,J=8.9Hz),8.09(dd,1H,J=2.2,8.9Hz),8,17( d,1H,J=2.2Hz).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap