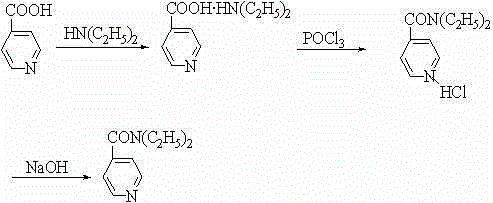
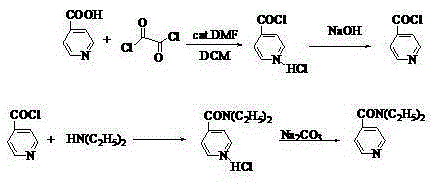
Method for preparing N,N-diethyl-3-pyridine carboxamide
A technology of pyridinecarboxamide and diethyl, which is applied in the field of medicinal chemistry, can solve the problems of unfavorable industrial production, high operation requirements, and high risk factor, and achieve the effects of improved production safety, high reaction yield, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Put 12.3g (about 0.1mol) of nicotinic acid and 128 ml of dichloromethane into the reaction bottle, stir, and put the reaction bottle into ice water to control the temperature at 0°C, and slowly add 12.8 ml of oxalyl chloride dropwise. , kept stirring at 0°C for 0.5~1h, then warmed up to room temperature, reacted for 4~6h, after the reaction was completed, 30% sodium hydroxide solution was added dropwise to adjust the pH of the reaction solution to 7, and then the temperature of the reaction mixture was controlled at -5~10°C to slowly Add 15.5ml of diethylamine dropwise. After the dropwise addition, react at room temperature for 6~8h. After the reaction is completed, add 17ml of 25% sodium carbonate solution. After stirring, let it stand for stratification, separate the lower water layer, and dry the organic layer. To obtain the crude product of N,N-diethyl-3-pyridinecarboxamide, add the crude product of N,N-diethyl-3-pyridinecarboxamide into dichloromethane, decolorize, oxi
Embodiment 2
[0021] Put 12.3g (about 0.1mol) of nicotinic acid and 160 ml of dichloromethane into the reaction bottle, stir, and put the reaction bottle into ice water to control the temperature at 0°C after stirring, and slowly add 15.4 ml of oxalyl chloride dropwise. , kept stirring at 0°C for 0.5~1h, then warmed up to room temperature, reacted for 4~6h, after the reaction was completed, 30% sodium hydroxide solution was added dropwise to adjust the pH of the reaction solution to 7, and then the temperature of the reaction mixture was controlled at -5~10°C to slowly Add 19.5ml of diethylamine dropwise. After the dropwise addition, react at room temperature for 6~8h. After the reaction is completed, add 20ml of 25% sodium carbonate solution. After stirring, let it stand for stratification. Separate the lower water layer and dry the organic layer. To obtain the crude product of N,N-diethyl-3-pyridinecarboxamide, add the crude product of N,N-diethyl-3-pyridinecarboxamide into dichloromethane, d
Embodiment 3
[0023] Put 12.3g (about 0.1mol) of nicotinic acid and 190 ml of dichloromethane into the reaction bottle, stir, and put the reaction bottle into ice water to control the temperature at 0°C after stirring, and slowly add 25 ml of oxalyl chloride dropwise. , kept stirring at 0°C for 0.5~1h, then warmed up to room temperature, reacted for 4~6h, after the reaction was completed, 30% sodium hydroxide solution was added dropwise to adjust the pH of the reaction solution to 7, and then the temperature of the reaction mixture was controlled at -5~10°C to slowly Add 30 ml of diethylamine dropwise. After the dropwise addition, react at room temperature for 6~8 hours. After the reaction is completed, add 33 ml of 25% sodium carbonate solution. After stirring, let stand to separate and separate the lower water layer, and dry the organic layer. To obtain the crude product of N,N-diethyl-3-pyridinecarboxamide, add the crude product of N,N-diethyl-3-pyridinecarboxamide into dichloromethane, deco
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap