3-O-(para-methanesulfonate benzyl)-ascorbic acid with anti-cancer activity and preparation method
A technology of mesylate and ascorbic acid, applied in the field of medical drugs, can solve the problems of single administration route, poor patient compliance, loss of activity, etc., achieve good clinical application value, and significantly inhibit activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1. Synthesis of 4-((methylsulfonyl)oxy)benzyl mesylate (Ms-HBA-ASA)
[0018] Add 25ml of dichloromethane into a 100ml three-necked flask equipped with a reflux condenser, turn on the constant temperature oil bath and magnetic stirrer, then add 2.5g of p-hydroxybenzyl alcohol (HBA), add 7ml of triethylamine, and heat Heat and stir until the p-hydroxybenzyl alcohol dissolves. 7.5 g of methanesulfonic anhydride (MsOMs) were dissolved in 15 ml of dichloromethane solution. The oil bath was removed and changed to an ice bath environment, and methanesulfonic anhydride dichloromethane solution was added dropwise. After the dropwise addition, remove the ice bath, change to 25 ° C oil bath heating, magnetic stirring reaction for 4 to 6 hours, after the reaction, the reaction solution was washed three times with water, the concentrated organic layer was collected, and column chromatography (petroleum ether: ethyl acetate = 2:1) separation, vacuum rotary evaporation, and con
Embodiment 2
[0019] Example 2. 3-O-(p-methylsulfonate benzyl)ascorbic acid (Ms-HBA-ASA)
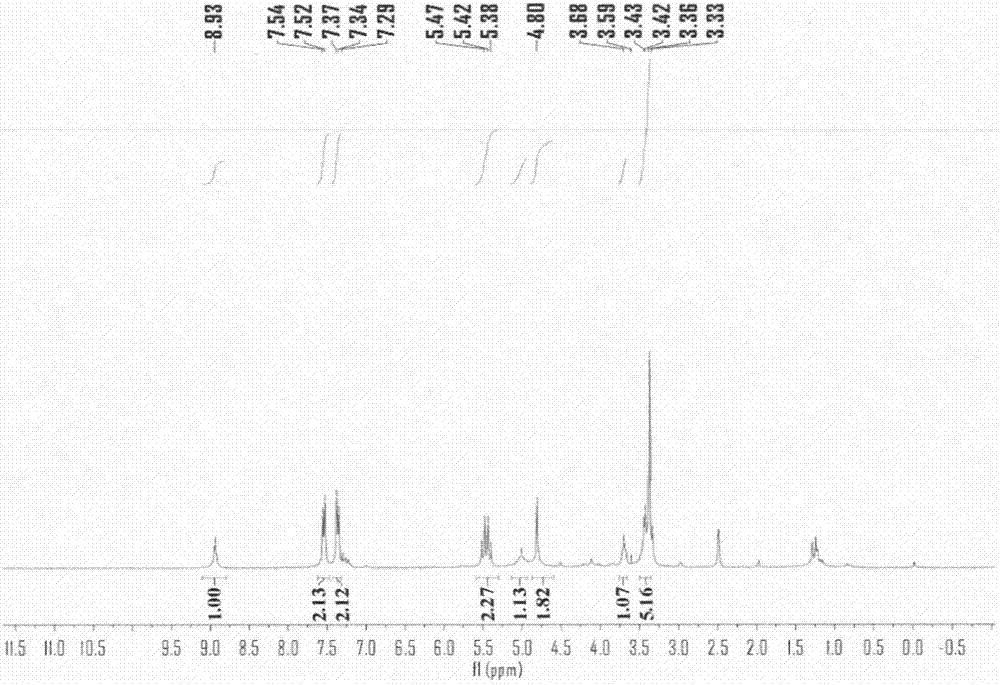
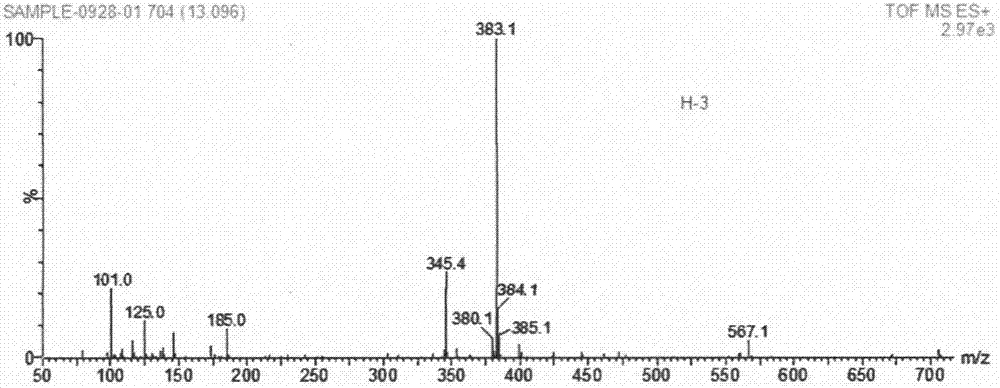
[0020] Add 25ml of ethanol to a 100ml three-neck flask, turn on the constant temperature oil bath and magnetic stirrer, add 2g of ascorbic acid, add 1.74ml of triethylamine, protect with nitrogen, then add 3.5g of Ms-HBA-Ms, and heat at 25°C Stir the reaction for 1 to 3 hours, separate the reaction solution by column chromatography (dichloromethane:methanol=30:1), evaporate in vacuo, and concentrate to obtain a white solid that is 3-O-(p-methylsulfonate benzyl)ascorbic acid (Ms-HBA-ASA) 3.52g, reaction yield 86%. The structure of the product was characterized by H NMR and mass spectrometry (see Figure 1-2 ).
Embodiment 3
[0021] Example 3. Cancer cell inhibition test of 3-O-(p-methylsulfonate benzyl) ascorbic acid
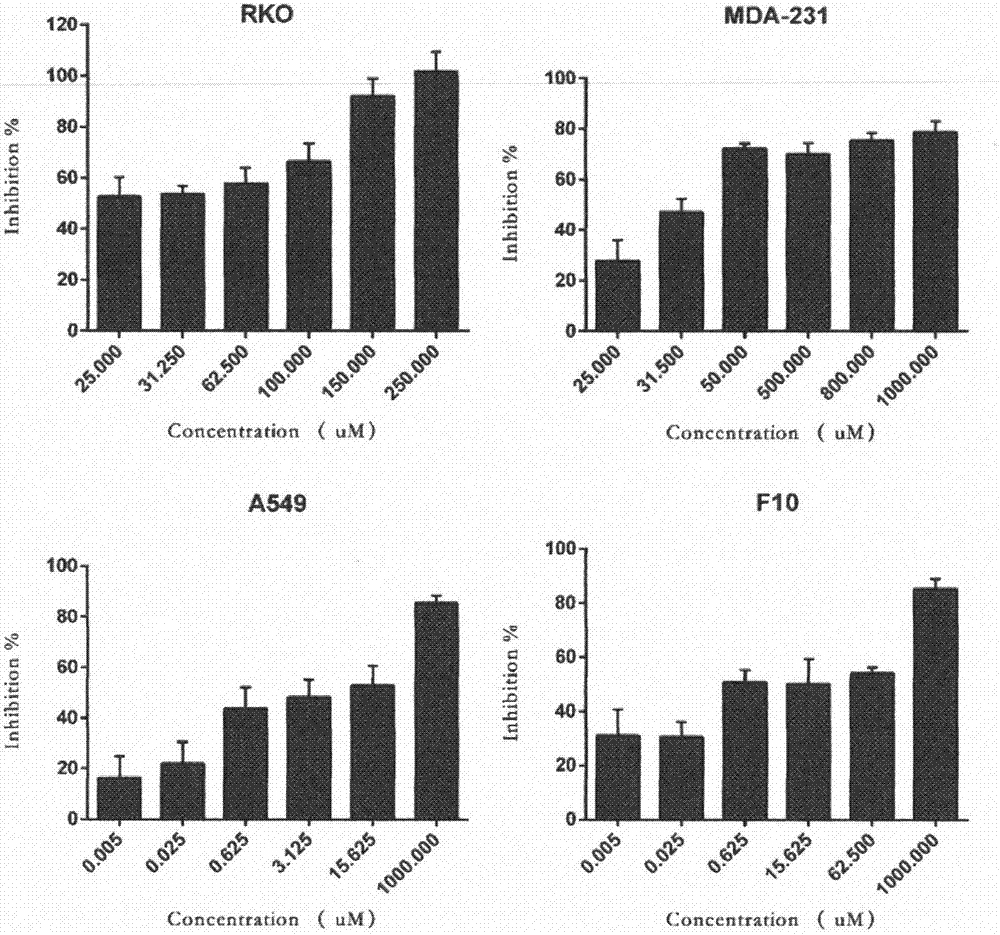
[0022] Cytotoxicity was evaluated by MTT method. Human colon cancer cell line RKO, human lung cancer cell line A549, human breast cancer cell line MDA-231 and human melanoma cell line B16-F10 were seeded in 96-well plates at appropriate concentrations, each well The total volume is 200ul. After culturing for 24 hours, discard the culture medium, add 200ul of culture medium with different drug concentrations, and set up a solvent control group at the same time. After 72 hours of drug action, add 50ul of 1mg / ml MTT solution to each well, continue to cultivate for 4h, and remove the culture medium. solution, add 150ul DMSO to each hole, and after the color is completely dissolved, measure the absorbance value with a wavelength of 490nm with an enzyme-linked analyzer, and calculate the inhibitory rate of the drug on cell growth (see image 3 ). Calculation of IC by Bliss method 50 valu
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap