Chemical quantitative analysis method for occurrence state of silver in ferro-manganese oxide ore
A technology of iron-manganese oxide ore and its occurrence state, which is applied in the field of analysis and detection, can solve the problems of targeted recovery and the inability to accurately measure the dispersed silver content, and achieve the effect of high accuracy and fast analysis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
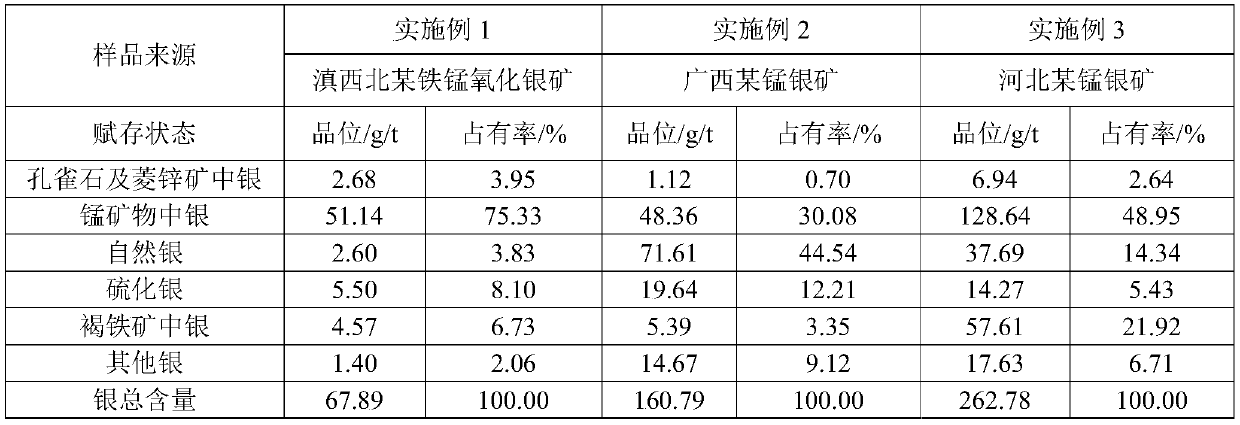
[0050] This embodiment provides a chemical quantitative analysis method for the silver occurrence state in iron-manganese oxide ore, and the process is as follows.
[0051] (1) Weigh 500g iron-manganese oxide ore (an iron-manganese silver oxide mine in northwest Yunnan), crush it to -0.074mm and account for 100%, and use the heap cone quartering method to mix and set aside;
[0052] (2) Weigh 2 g of the crushed ore in step (1), place it in a 100 mL beaker, and add 50 mL of concentrated ammonia water (15%, if not specified, % refers to mass fraction) to prepare a concentration of 150 g / L Ammonium carbonate solution was shaken at normal temperature for 2 hours, and the filter residue was collected after filtration, and the filtrate was measured by atomic absorption spectrometer for the dispersed silver in malachite and smithsonite;
[0053] (3) add 50mL of 50g / L hydroxylamine hydrochloride-150g / L sodium chloride solution to the filter residue collected in the previous step fo
Embodiment 2
[0059] This embodiment provides a chemical quantitative analysis method for the silver occurrence state in iron-manganese oxide ore, and the process is as follows.
[0060] (1) Weigh 500g iron-manganese oxide ore (a manganese-silver mine in Guangxi), pulverize to -0.074mm and account for 100%, and use heap cone quartering method to mix and set aside;
[0061] (2) Take by weighing 2 g of the crushed ore in step (1), place it in a 100 mL beaker, add 50 mL of concentrated ammonia water (28%) to prepare an ammonium carbonate solution of 100 g / L, vibrate at room temperature for 2 hours, and collect the filter residue after filtration , the filtrate adopts atomic absorption spectrometer to measure the dispersed silver in malachite and smithsonite;
[0062] (3) add 50mL of 40g / L hydroxylamine hydrochloride-100g / L sodium chloride solution to the filter residue collected in the previous step for leaching, filter after shaking for 15min, and wash with 10g / L hydroxylamine hydrochlor
Embodiment 3
[0068] This embodiment provides a chemical quantitative analysis method for the silver occurrence state in iron-manganese oxide ore, and the process is as follows.
[0069] (1) Weigh 500g iron-manganese oxide ore (a manganese-silver mine in Guangxi), pulverize to -0.074mm and account for 100%, and use heap cone quartering method to mix and set aside;
[0070] (2) Take by weighing 2 g of the crushed ore in step (1), place it in a 100 mL beaker, add 50 mL of concentrated ammonia water (15%) to prepare an ammonium carbonate solution of 200 g / L, vibrate at room temperature for 2 hours, and collect the filter residue after filtration , the filtrate adopts atomic absorption spectrometer to measure the dispersed silver in malachite and smithsonite;
[0071] (3) add 50mL of 60g / L hydroxylamine hydrochloride-200g / L sodium chloride solution to the filter residue collected in the previous step for leaching, filter after shaking for 15min, and wash with 10g / L hydroxylamine hydrochlor
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap