MODIFIED AMADORIASE AND METHOD FOR PRODUCING THE SAME, AGENT FOR IMPROVING SURFACTANT RESISTANCE OF AMADORIASE AND COMPOSITION FOR MEASURING HbA1c USING THE SAME
a technology of amadoriase and surfactant resistance, applied in the field of amadoriase excellent, can solve the problems of inability to report amadoriases having high surfactant resistance, and inability to obtain accurate measurement values, etc., to achieve excellent surfactant resistance and reduce residual activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
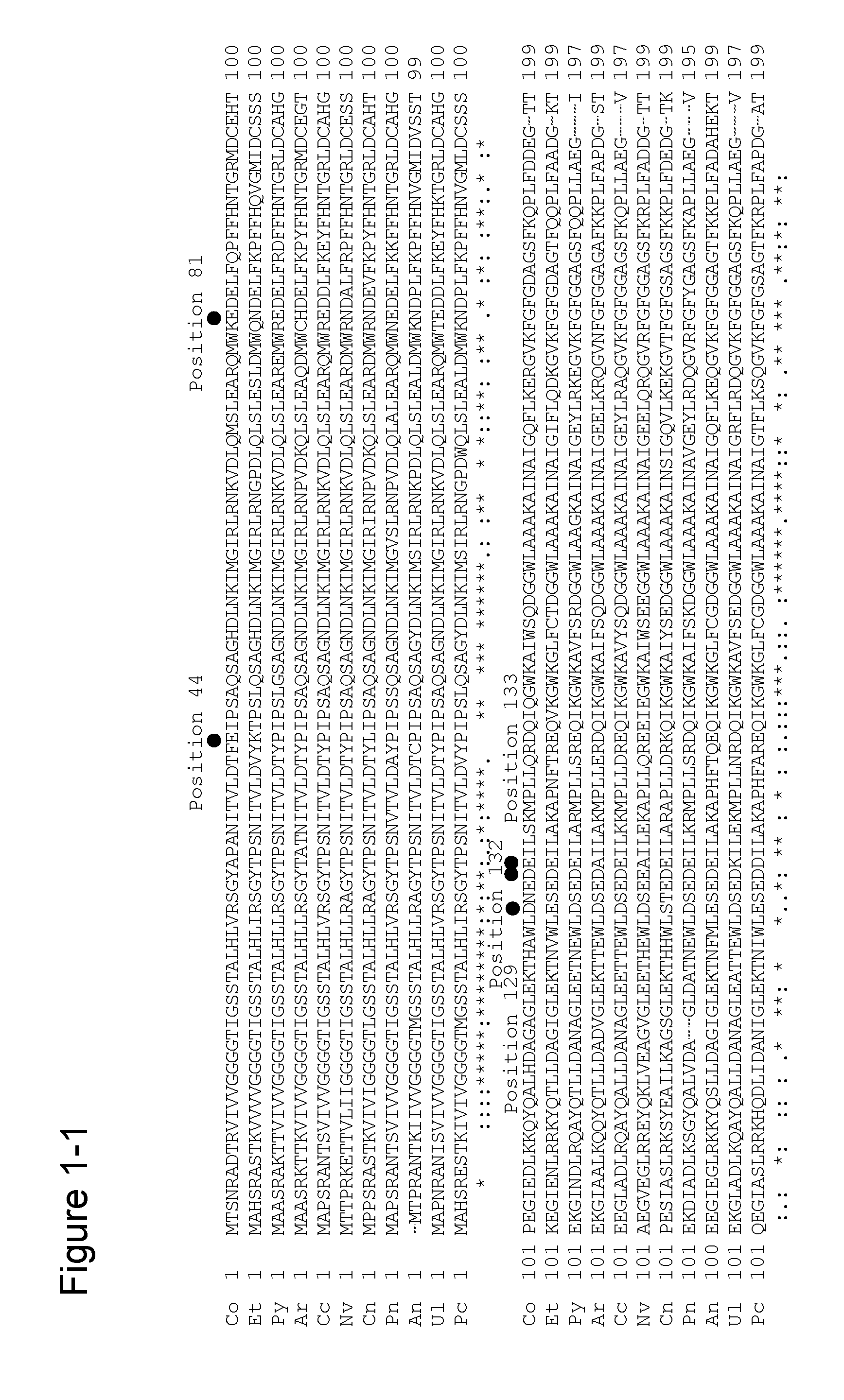
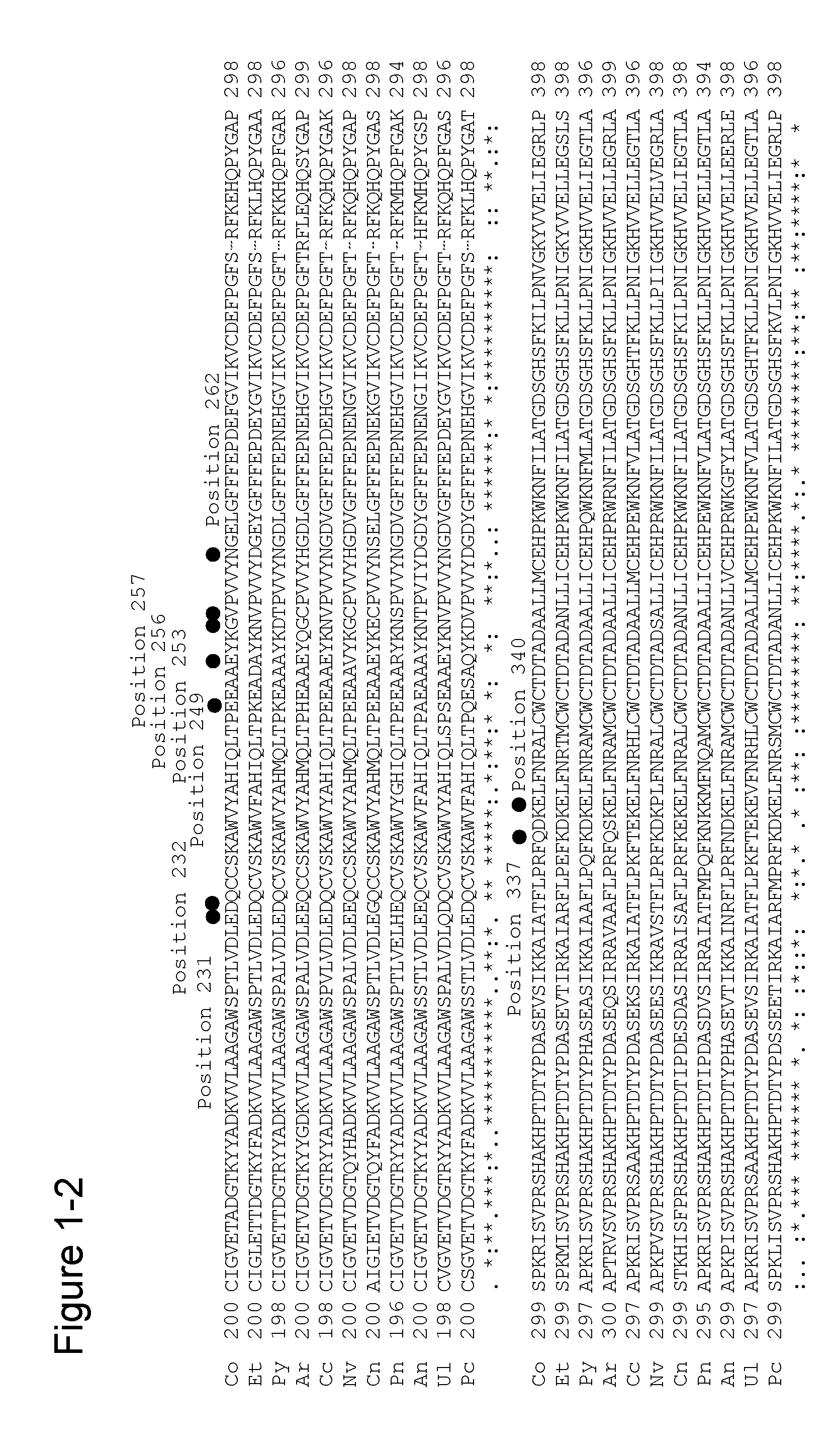
Mutation(s) for Improved Surfactant Resistance
[0294](1) Preparation of Recombinant Plasmid pKK223-3-CFP-T7 DNA
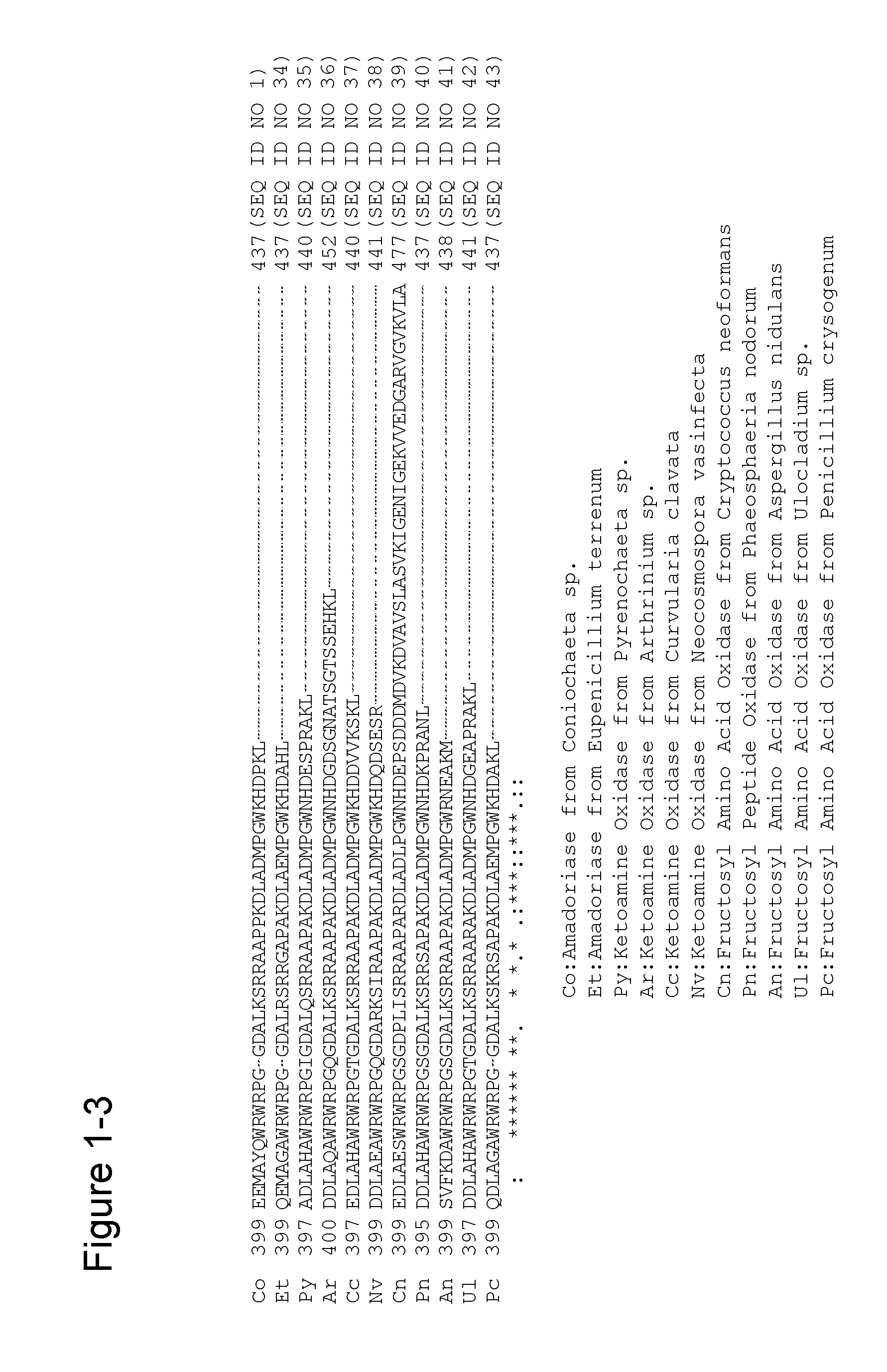
[0295]Escherichia coli strain JM109 (pKK223-3-CFP-T7) having a recombinant plasmid containing CFP-T7 gene (SEQ ID NO: 2) (see International Publication No. WO 2007 / 125779) was inoculated in 2.5 ml of LB-amp medium [1% (w / v) bactotrypton, 0.5% (w / v) peptone, 0.5% (w / v) NaCl, and 50 μg / ml ampicillin] and subjected to shake culture at 37° C. for 20 hours and a culture product was obtained. [0160]1
[0296]The culture product was centrifuged at 7,000 rpm for 5 minutes to collect strains. Then the recombinant plasmid pKK223-3-CFP-T7 was extracted and purified therefrom using the QIAGEN tip-100 kit (QIAGEN), and 2.5 μg DNA of the recombinant plasmid pKK223-3-CFP-T7 was obtained.
(2) Site-Directed Modification Operation of DNA of Recombinant Plasmid pKK223-3-CFP-T7
[0297]PCR was carried out under conditions described below using obtained DNA of the recombinant plasmid pKK223-3-CF
example 2
Accumulation of Mutation for Improved Surfactant Resistance
[0336]Based on the findings of mutations for enhancing surfactant resistance obtained in Example 1, multiple variants (a double variant, a triple variant, a quadruple variant, a quintuple variant, a sextuple variant, or a septuple variant) were tested to combine and accumulate these mutations in order to obtain an amadoriase having further increased surfactant resistance.
[0337]SEQ ID NO: 3 is the amino acid sequence of an amadoriase derived from the genus Coniochaeta into which a mutation for improving substrate specificity (E98A) and mutations for enhancing heat stability (F43Y, G184D, deletion of 3 carboxy-terminal amino acid residues) were introduced (hereinafter indicated with “CFP-D”), and is encoded by the gene of SEQ ID NO: 4. Mutations for enhancing surfactant resistance were accumulated using plasmid DNA in which CFP-D gene was inserted into pKK223-3 vector as a template. PCR reaction was carried out under the same con
example 3-1
Evaluation for Surfactant TTAC
[0347]Tetradecyltrimethylammonium chloride (hereinafter indicated with “TTAC”) was used in place of the surfactant CTAC used in Example 2 to evaluate the stability of CFP-D. The surfactant resistance of various modified amadoriases was evaluated in accordance with a measurement method for surfactant resistance according to Example 1, although under surfactant treatment conditions in which the amadoriases were each diluted in a 20 mM potassium phosphate buffer solution (pH 7.0) and mixed with 0.04% TTAC. The results are shown in Table 3-1. In this respect, it was confirmed that when the warmed sample was again measured for activity 30 minutes after 2-fold dilution in a BSA solution, there was no change in the activity value.
TABLE 3-1Plasmid asAmino AcidSEQ ID NO ofResidualPlasmidTemplateEnzymeMutationOligonucleotide UsedActivity (%)pKK223-3-NoneCFP-DNoneNone29.2CFP-DpKK223-3-pKK223-3-CFP-D1E44P23.2843.2CFP-D1CFP-DpKK223-3-pKK223-3-CFP-D2E44P / E340P16.1769.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap