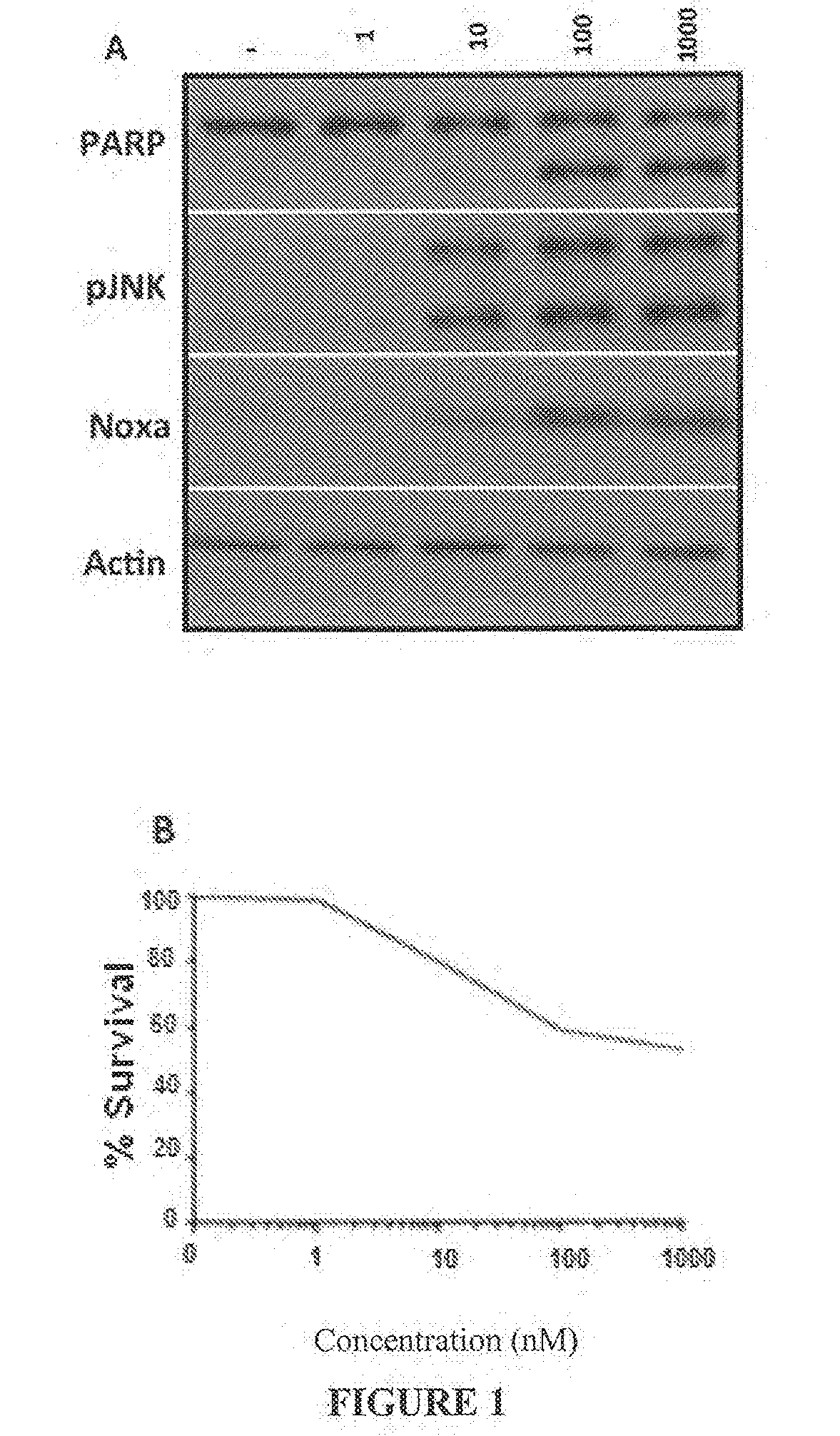
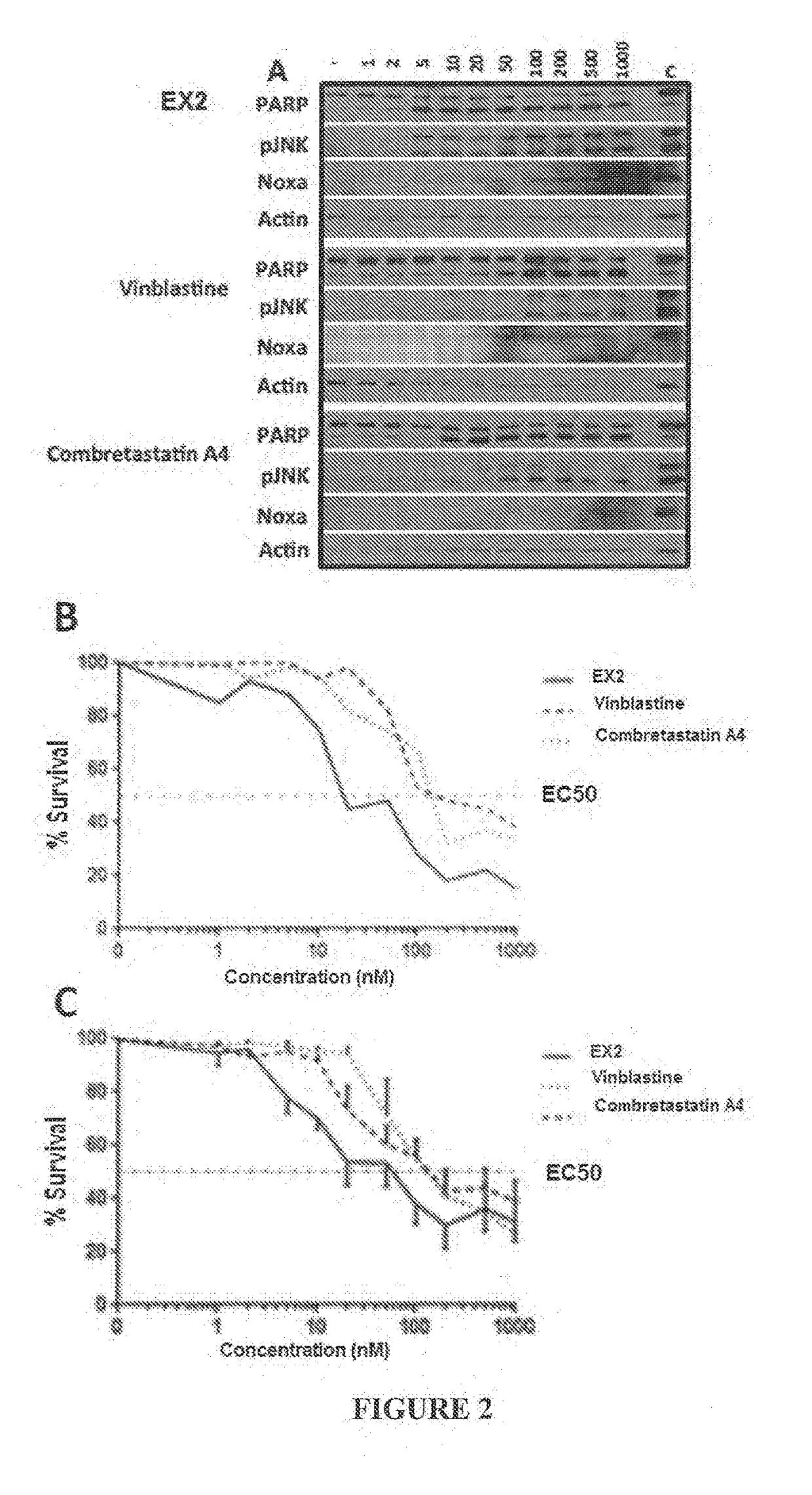
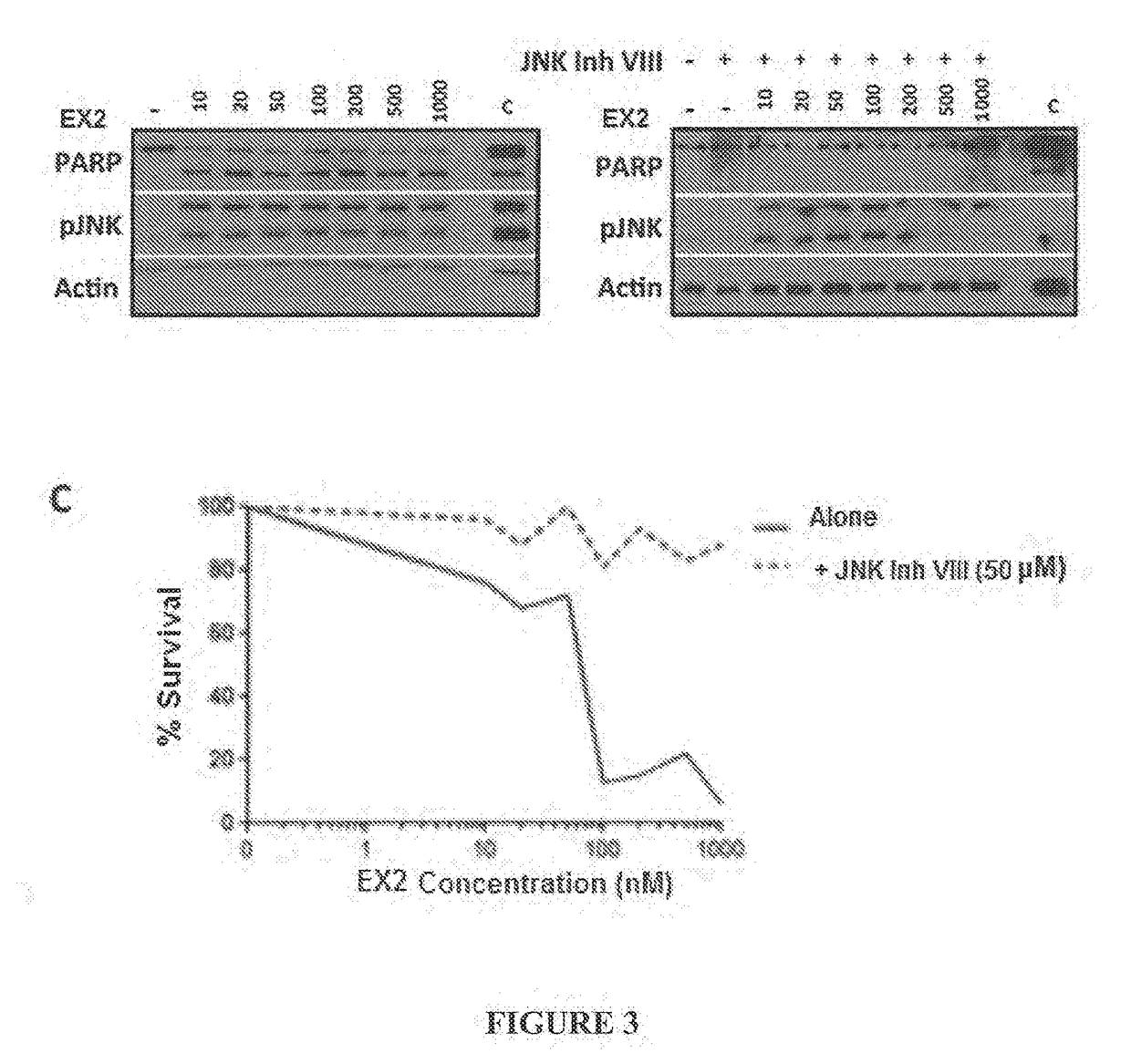
Combination treatment protocol
a technology of lymphocytic leukemia and treatment protocol, which is applied in the field of combination therapy for chronic lymphocytic leukemia, can solve the problems of rare complete remission, ibrutinib therapy is susceptible to formula compounds, and the cells which leave the stromal niche are not easy to recur, so as to achieve more resistance to drugs, higher potency, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-Methyl-7-hydroxy-3-(3,4,5-trimethoxybenzoyl)-6-methoxybenzofuran
[0101]
[0102]Preparation A
[0103]To a stirred solution of 2-Bromo-7-acetoxy-3-(3,4,5-trimethoxybenzoyl)-6-methoxybenzofuran (20 mg, 0.042 mmol), methyl-boronic acid (40 mg, 0.67 mmol), in 1,4-dioxane (2 mL) at 90° C. was added tetrakis-triphenylphosphine palladium (11 mg, 0.01 mmol) followed by the addition of a solution of sodium bicarbonate (40 mg, 0.48 mmol) in distilled water (0.5 mL). The reaction mixture turned red after 5 minutes. After 2 hours (tlc) the reaction mixture was brought to room temperature and was added saturated ammonium chloride (2 mL) and diluted with dichloromethane (20 mL). The organic layer was separated and washed with water, dried over magnesium sulfate and the solvent was removed by distillation under vacuum. The residue was purified by PTLC (eluent=Dichloromethane / Methanol, 1:1) to give the title compound (acetate cleaved during reaction) as a fluffy white solid; (3 mg, 19%).
[
example 2
Preparation of Disodium 6-methoxy-2-methyl-3-(3,4,5-trimethoxybenzoyl)benzofuran-7-yl phosphate
[0106]
[0107]Step 1: Dibenzyl 6-methoxy-2-methyl-3-(3,4,5-trimethoxybenzoyl)benzofuran-7-yl phosphate:
[0108]To a mixture of 0.081 g (0.22 mmol) of (7-hydroxy-6-methoxy-2-methylbenzofuran-3-yl)(3,4,5-trimethoxyphenyl)methanone, 0.086 g (0.261 mmol) of carbon tetrabromide and 0.063 ml (0.283 mmol) of dibenzylphosphite in 2.5 ml of anhydrous acetonitrile 0.046 ml of anhydrous triethylamine was added dropwise at 0° C. under nitrogen atmosphere. The resulting mixture was stirred for 2 h at room temperature, then diluted to 20 ml with ethyl acetate, washed with water brine, dried over anhydrous magnesium sulfate, filtered off and evaporated to dryness under reduced pressure. The residue was purified by flash column chromatography (dichloromethane / ethyl acetate, 9:1) to give the title compound as a colorless foam (0.13 g, 94%); 1H NMR (CDCl3) δ 2.42 (s, 3H, Me-2); 3.83 (s, 1H, OMe); 3.93 (s, 3H, OM
example 3
Phase 1b of Ibrutinib and Example 2 in Patients with Relapsed or Refractory CLL
[0135]A phase 1b trial is conducted. Table 1 provides a summary of the trial conditions. A list of abbreviations used in this Example is provided at the end of the Example. Example 2 is an example of a compound of Formula (I). The study is also capable of variation such a providing the patient first with ibrutinib followed by exposure to Example 2. Such a variation is to be taken into account during the following discussion of this one, non-limiting, embodiment.
TABLE 1Summary of trialA Phase Ib Study of Example 2 andIbrutinib in Patients with Relapsed / TitleRefractory Chronic Lymphocytic LeukemiaShort TitleEXAMPLE 2 and ibrutinib in CLLPhaseIbMethodologyInterventional studyStudy Duration24 monthsObjectivesTo study the safety and efficacy of EXAMPLE2 in combination with ibrutinib in patients with CLLNumber of SubjectsUp to 27 patientsDiagnosis and MainPatients with CLLInclusion CriteriaStudy Product,Study pr
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap