Pharmaceutical combination comprising lsz102 and alpelisib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
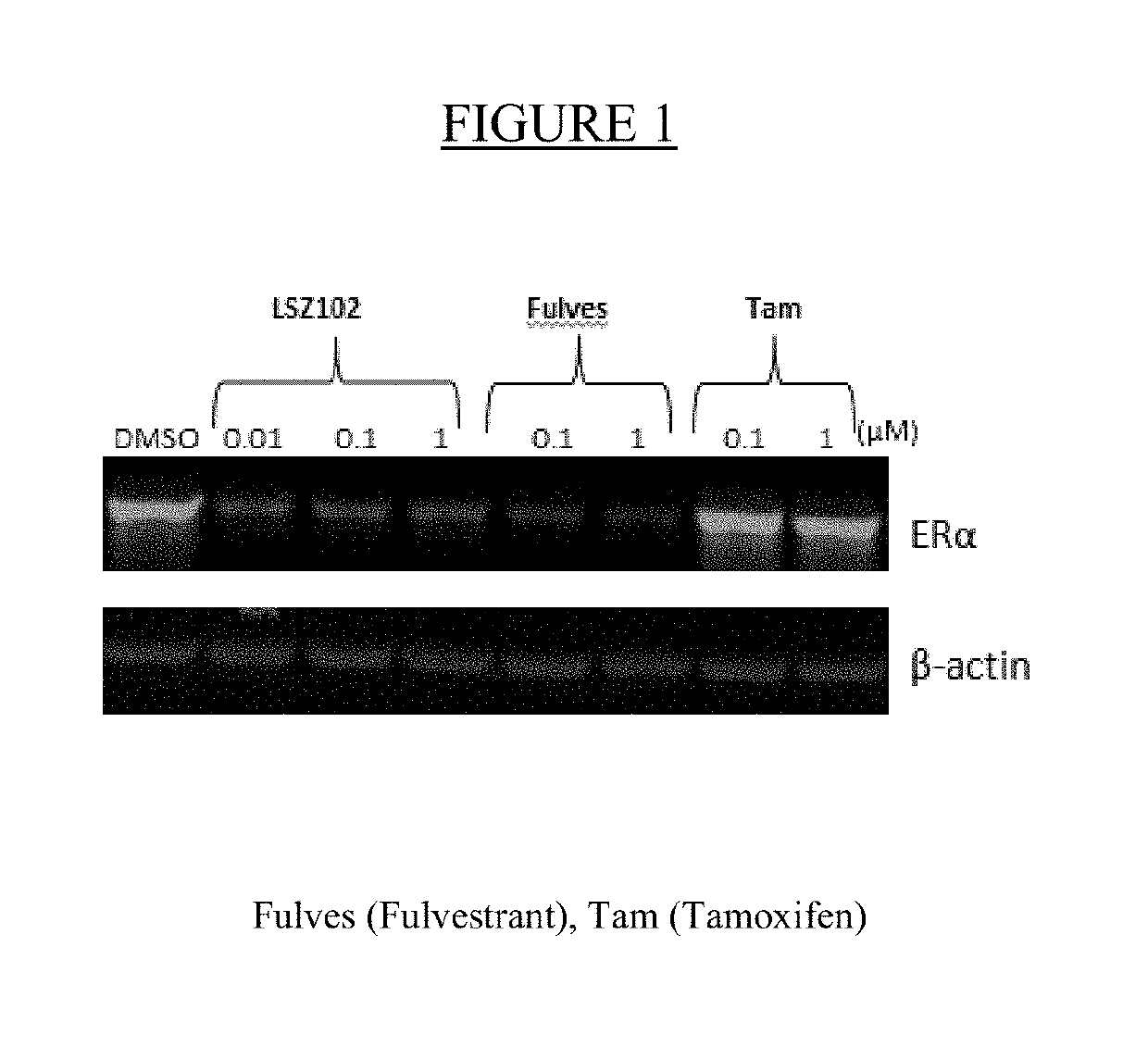
LSZ102 promotes ER degradation in MCF-7 cells
[0108]Western blot. For the analysis of LSZ102, fulvestrant, and tamoxifen on ERα protein levels in MCF-7 tumors at the end of efficacy study, snap frozen tumors were pulverized into a powder and then transferred to Lysing Matrix Tubes (MP Biomedicals Cat. #6913-500) mixed with cold lysis buffer (1× cell lysis buffer; Cell Signaling, Cat.#9803S) containing Complete Mini (1 tablet to 10 mL), PhosStop (1 tablet to 10 mL and 1 M Urea) homogenized by a Fast Prep 24 Tissue Lyser (MP Biomedicals). Total protein concentrations of the lysate were tested by BCA assay (Pierce BCA Protein Assay Kit, Prod #23225, Thermo Scientific) according to the manufacturer's instructions. Lysates were separated by SDS-PAGE, transferred onto membranes, and then immunoblotted using an anti-ERα antibody (Santa Cruz Biotechnology, HC-20), as well as an anti-tubulin antibody as a loading control. Western blots were scanned for quantification of the immunoblotted bands.
example 2
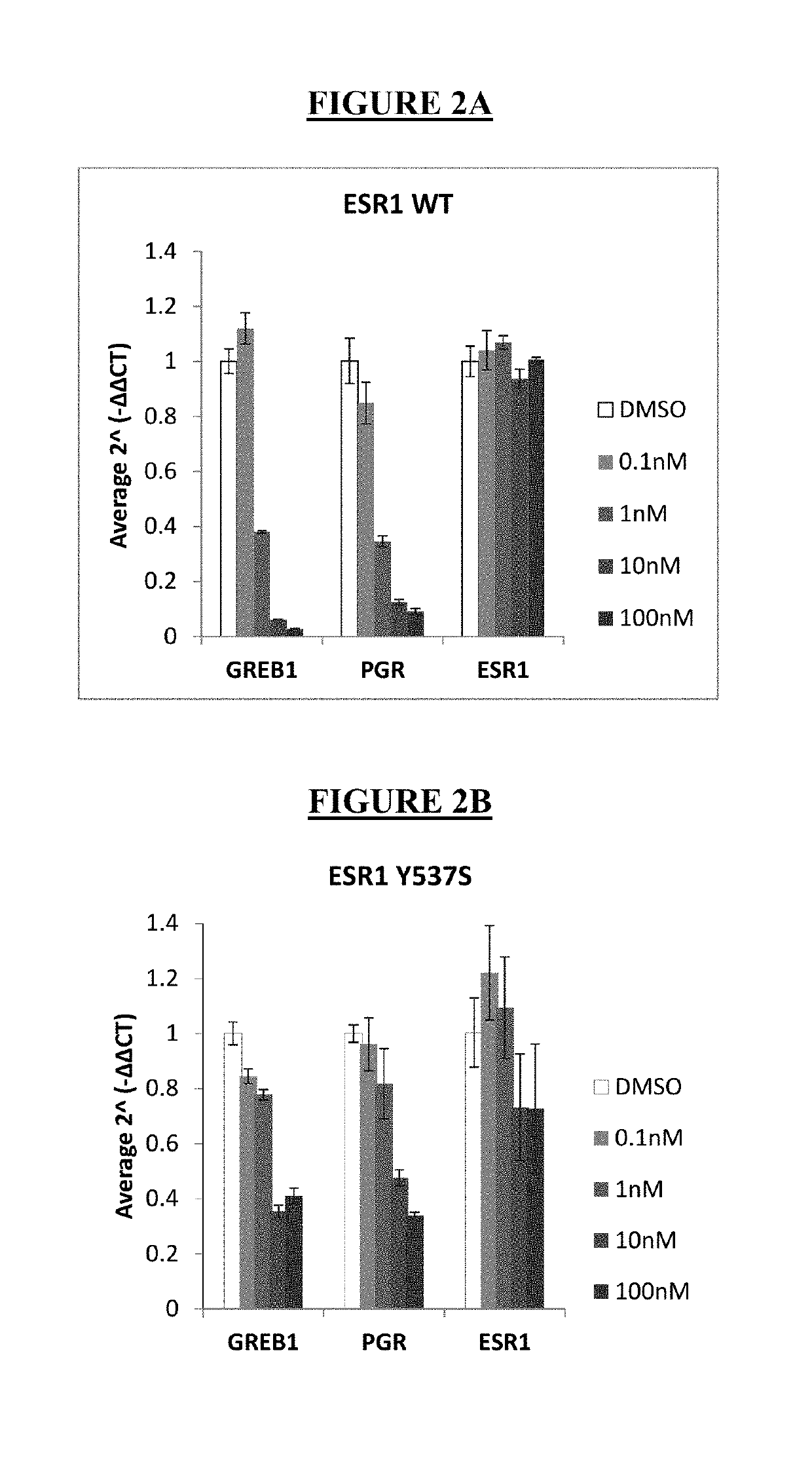
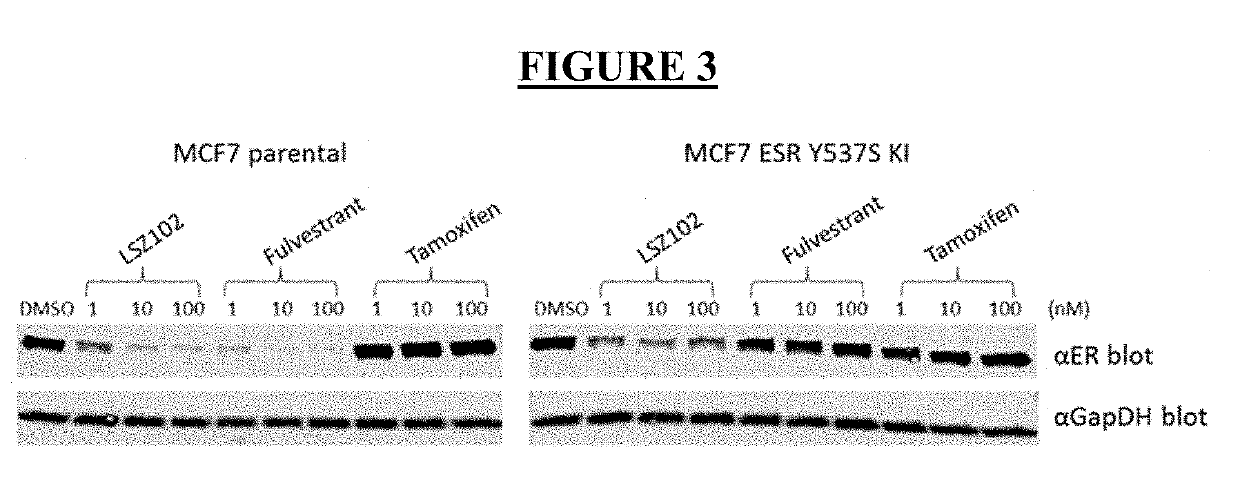
LSZ102 Anti-Proliferation and ER Degradation Activity in MCF-7 Parental and Y537S Cells
[0109]The effects of LSZ102, fulvestrant, and tamoxifen as single agents were studied in MCF-7 parental (ER wildtype, or WT) and ER Y537S mutant cells. MCF-7 WT cells and Y537S mutant cells were incubated in RPMI (without phenol red) plus 10% charcoal dextran-stripped serum and treated with escalated concentration of compounds in the presence of 0.1 nM (nanomolar) estradiol (WT) or no estradiol (Y537S). Cell viability was determined by CellTiter-Glo (CTG) assay after 7 days of compound treatment. For ERE-luciferase assay, cell luciferase signal was measured using Bright-Glo assay after 24 hours. The IC50 value is the compound concentration which inhibits 50% of the CTG signal by 50%. IC50 nM values were calculated using the XLfit software and are defined as the inflection point of the fitted inhibition curves. The results for anti-proliferation activity of LSZ102, fulvestrant, and tamoxifen in MCF-7
example 3
MCF-7 Xenograft Model in NSG Mice
[0113]The estrogen response ER positive (ER+) MCF-7 cell line was shown to be sensitive to LSZ102 in vitro. To demonstrate targeted anti-tumor activity in the orthotopic MCF-7 xenograft model in NOD scid gamma (NSG) mice, 1, 3, 10 and 20 mg / kg of LSZ102 was administered orally (PO) once daily (QD) along with 5 mg of fulvestrant administered subcutaneously (SC) once weekly (Qweek) per mouse and 60 mg / kg of tamoxifen administered orally (PO) once daily for 5 days per week as positive controls (FIG. 4). Mice were supplemented with estradiol (0.72 mg estradiol / 90-day release pellets) to further support MCF-7 tumor growth several days prior to cell implantation. MCF-7 tumors were established in female NSG mice by injection of 10×106 cells in 50% Matrigel® into the axillary mammary fat pad area of each mouse. When tumors reached an average of 200 mm3, mice were randomized according to tumor volume into treatment groups (n=8). The effect of the treatment
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass flow rate | aaaaa | aaaaa |
| Mass flow rate | aaaaa | aaaaa |
| Mass flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap