Preparation method of substituted 1,3,4-diazole-2-(3H)-ketone
A representative and compound technology, applied in the field of preparation of 1,3,4-diazol-2-one, can solve the problems of inconvenient reaction process, unsafe operation, hidden dangers in storage, transportation and use, etc. Convenience, ease of use, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
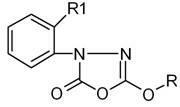
[0027] Example 1 Preparation of 5-methoxy-3-(2-methoxyphenyl)-1,3,4-oxadiazol-2-(3H)-one Ⅰ
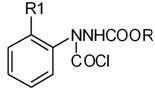
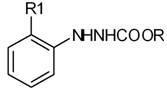
[0028] In a 2000ml four-necked flask equipped with a condenser, put 800ml of toluene solution containing 88.8g of triphosgene (0.3mol) into it, start stirring, and then add 196g of 2-methoxyphenyl-3-carbazinic acid Methyl ester (1 mol). Then heat up to 70°C to react until no HCl gas is released violently, then raise the temperature to 90°C and keep it warm for 2 hours, then replace the -10°C chilled water in the condenser with room temperature circulating water, and continue the reaction until no more bubbles are released. Stop responding. After cooling to room temperature, negative pressure precipitation removes toluene, precipitation final temperature 70 ℃, vacuum degree 20mmHg, obtain 2-methoxyphenyl-3-chloroformyl-3-carbazinic acid methyl ester of 258.5g, without It was directly used in the next reaction after treatment.
[0029] Dissolve the above product in 800ml of dichlorometh
Embodiment 2
[0030] Example 2 Preparation of 5-methoxy-3-(2-methoxyphenyl)-1,3,4-oxadiazol-2-(3H)-one Ⅱ
[0031] In a 1000ml four-necked flask equipped with a condenser, add 500ml of benzene, 59.2g of triphosgene (0.2mol), and 98g of methyl 2-methoxyphenyl-3-carbazinate (0.5mol) in sequence, and start stirring slowly Heat up the temperature, add the above solution dropwise into the four-neck flask within 30 minutes, then heat up to 40°C for reaction until no HCl gas is released violently, then raise the temperature to 70°C for 1 hour and keep the reaction for 1 hour, then put the condenser at -10°C Replace the chilled water with room temperature circulating water, continue the reaction until no more bubbles are released, and stop the reaction. After cooling to room temperature, benzene was removed by precipitation under negative pressure. The final temperature of the precipitation was 50° C., and the vacuum degree was 20 mmHg to obtain 133.5 g of 2-methoxyphenyl-3-chloroformyl-3-carbazinic
Embodiment 3
[0033] Example 3 Preparation of 5-methoxy-3-(2-ethoxyphenyl)-1,3,4-oxadiazol-2-(3H)-one
[0034] In a 2000ml four-necked flask equipped with a condenser, add 1000ml xylene, 148g triphosgene (0.5mol), and 210g methyl 2-ethoxyphenyl 3-carbazate (1mol) in sequence. Stir at 20°C for 10 minutes, then heat up to 90°C for reaction until no HCl gas is released violently, then raise the temperature to 120°C for 30 minutes, then replace the -10°C chilled water in the condenser with room temperature circulating water to continue the reaction Until no more bubbles are released, stop the reaction. After cooling to room temperature, xylene was removed by negative pressure precipitation, the final temperature of precipitation was 70°C, and the vacuum degree was 20mmHg to obtain 272.5g of methyl 2-ethoxyphenyl-3-chloroformyl-3-carbazate. It was directly used in the next reaction without treatment.
[0035] Dissolve the above product in 800ml of dichloromethane, then add 140ml of pyridine dro
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap