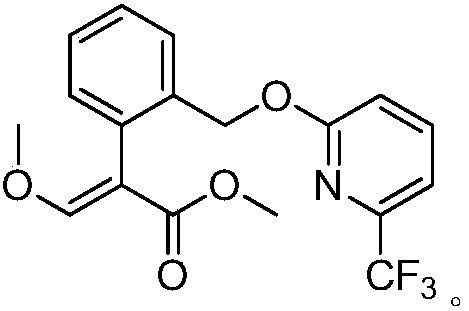
Preparation method of picoxystrobin
A technology of picoxystrobin and methoxy groups, applied in the field of preparation of picoxystrobin, can solve the problems of mass use of organic solvents, unfavorable industrial production, low purity of intermediates, etc., and achieves reduction of three wastes, reduction of material cost and solid state. The amount of waste generated, the effect of simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
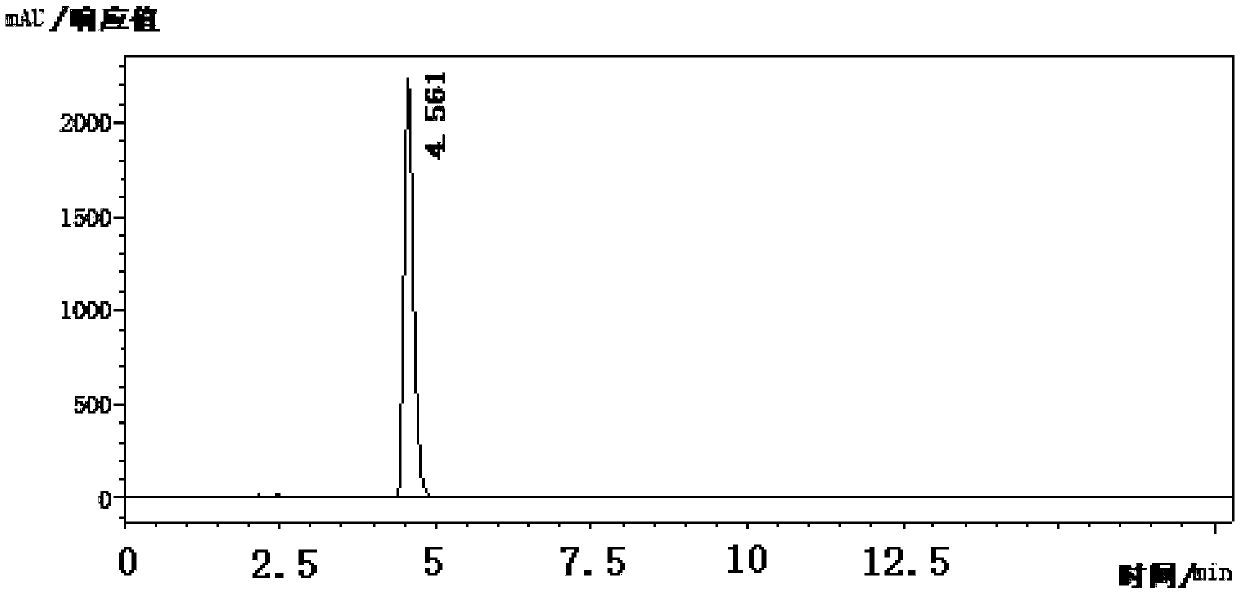
[0053] Add 160g sodium hydroxide aqueous solution (10%w / w), 36.7g 2-chloro-6-trifluoromethylpyridine (content 99%) in 500mL stainless steel autoclave, close still cover, oil bath is heated up to 150 ℃, The pressure of the kettle is 0.4MPa, after stirring and reacting for 5 hours, remove the heat source, take a sample, and control in the high performance liquid chromatography (condition: Agilent ZORBAX Eclipse XDB-C18, 4.6*250*5μm; mobile phase: methanol: 0.1% phosphoric acid = 55:45 (V:V); flow rate: 1.0mL / min; wavelength: 254nm), the central control spectrum is as follows figure 1 shown. After the reaction was complete, the pressure relief valve was opened, the reaction solution was cooled to 5° C., filtered and drained, the wet weight of the filter cake was 37.3 g, and the content was 92.7% by the external standard method (using 2-hydroxyl-6-trifluoromethylpyridine Sodium salt), the separation yield is 93.4%.
[0054] Add 37.3g of 2-hydroxyl-6-trifluoromethylpyridine sodi
Embodiment 2
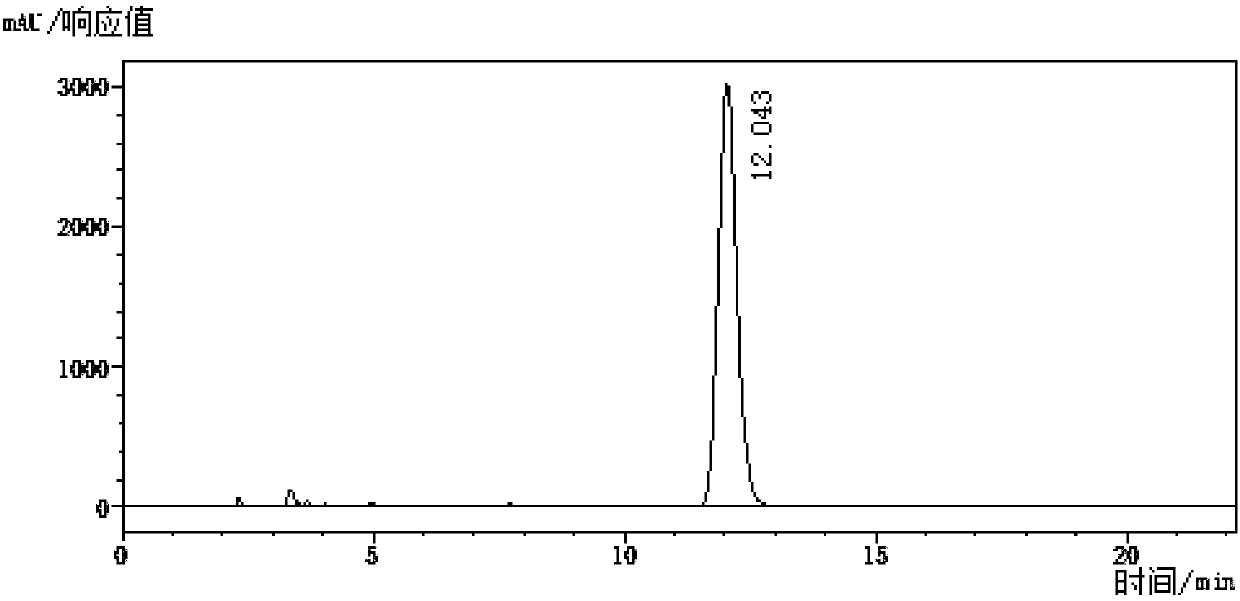
[0057] Add 40g aqueous sodium hydroxide solution (20% w / w), 33.7g 2-fluoro-6-trifluoromethylpyridine (content 98%), and 1.5g tetrabutylammonium bromide into a 250mL stainless steel autoclave. Close the lid of the kettle, raise the temperature of the oil bath to 160°C, and the pressure of the kettle to 1.2MPa. After stirring and reacting for 10 hours, remove the heat source, take a sample, and control in the high performance liquid chromatography (condition: Agilent ZORBAX Eclipse XDB-C18, 4.6*250*5μm; flow Phase: methanol: 0.1% phosphoric acid = 55:45 (V:V); flow rate: 1.0 mL / min; wavelength: 254 nm). After the reaction was complete, the pressure relief valve was opened, the reaction solution was cooled to 10°C, filtered and drained, the wet weight of the filter cake was 44.6g, and the content was 79.4% detected by the external standard method (with 2-hydroxyl-6-trifluoromethylpyridinium sodium Salt meter), the separation yield is 94.9%.
[0058] Add 44.6g of 2-hydroxyl-6-tr
Embodiment 3
[0060] In 250mL stainless steel autoclave, add 60g sodium hydroxide aqueous solution (40%w / w), 36.7g 2-chloro-6-trifluoromethylpyridine (content 99%), close still lid, oil bath is heated up to 140 ℃, The pressure of the kettle is 0.35MPa, after stirring and reacting for 3 hours, remove the heat source, take a sample, and control in the high performance liquid chromatography (condition: Agilent ZORBAX Eclipse XDB-C18, 4.6*250*5μm; mobile phase: methanol: 0.1% phosphoric acid = 55:45 (V:V); flow rate: 1.0 mL / min; wavelength: 254 nm). After the reaction is complete, open the pressure relief valve, cool the reaction solution to 10°C, filter and drain, the wet weight of the filter cake is 38.1g, and the content is 92.8% by the external standard method (with 2-hydroxyl-6-trifluoromethylpyridinium sodium Salt meter), the separation yield is 95.6%.
[0061] In a 500mL three-necked flask, add 38.1g of the wet product of 2-hydroxy-6-trifluoromethylpyridine sodium salt obtained in the
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap