1-carboxy alkyl-4-nitroimidazole preparation method and application thereof
A technology of nitroimidazole and carboxyalkyl, applied in the field of biochemical industry, can solve the problems of expensive equipment, time-consuming, troublesome processing, etc., and achieve the effects of simple and effective synthesis steps and high purity of products after identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
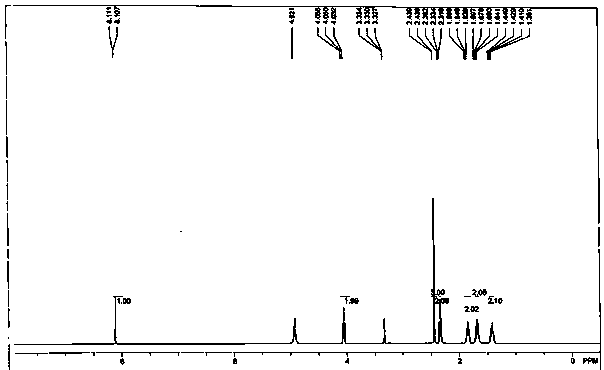
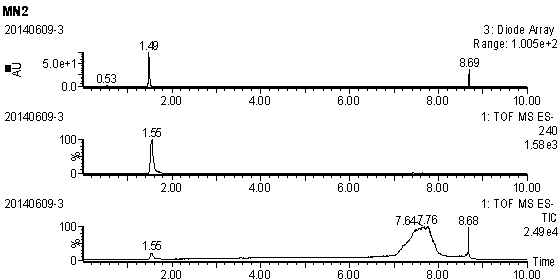
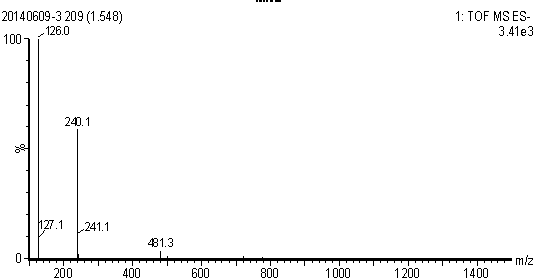
Embodiment 1
[0020] (1) Preparation of methyl 6-bromohexanoate: Add 1.95 g of 6-bromohexanoic acid (10 mmol) into 10 mL of methanol, slowly add thionyl chloride (15 mmol) dropwise under stirring in an ice bath, and then raise the temperature to reflux , Keep reflux for 2-3h. After the reaction was completed, spin-dry by vacuum rotary evaporation, then add methanol to spin-dry and repeat twice (10 mL / time, 2 times) to obtain the compound 6-bromohexanoic acid methyl ester, which is set aside.
[0021] (2) Preparation of 2-methyl-4-nitro-1-(6-carboxyhexyl)imidazole ester: 1.27 g of 2-methyl-5-nitroimidazole (10 mmol) was dissolved in 10 mL of N,N- In dimethylformamide (DMF), add sodium hydride (60% content, 480 mg, 12 mmol) under ice bath, stir for 10 min, remove the ice bath, return to room temperature and stir for 1 h, add methyl 6-bromohexanoate (10 mmol, dissolved in 2 mL DMF), stirred overnight at room temperature, and quenched with saturated ammonium chloride. Extracted twice with dich
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap