Low-alkali or alkali-free active bright-blue dye and preparation method thereof
A technology of reactive brilliant blue and dyes, which is applied in the direction of reactive dyes, azo dyes, organic dyes, etc., can solve the problems of high energy consumption, labor consumption, and waste water, and achieve the reduction of waste water discharge, energy saving, and good dryness. The effect of wet fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
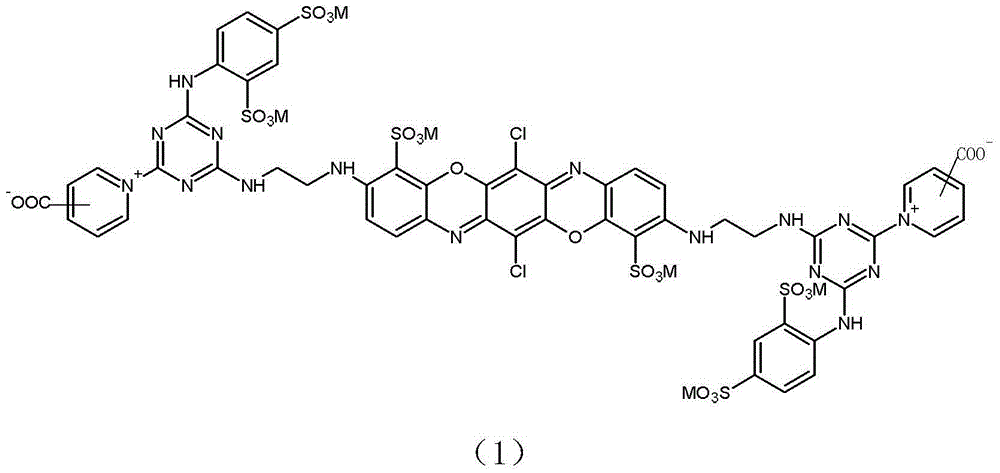
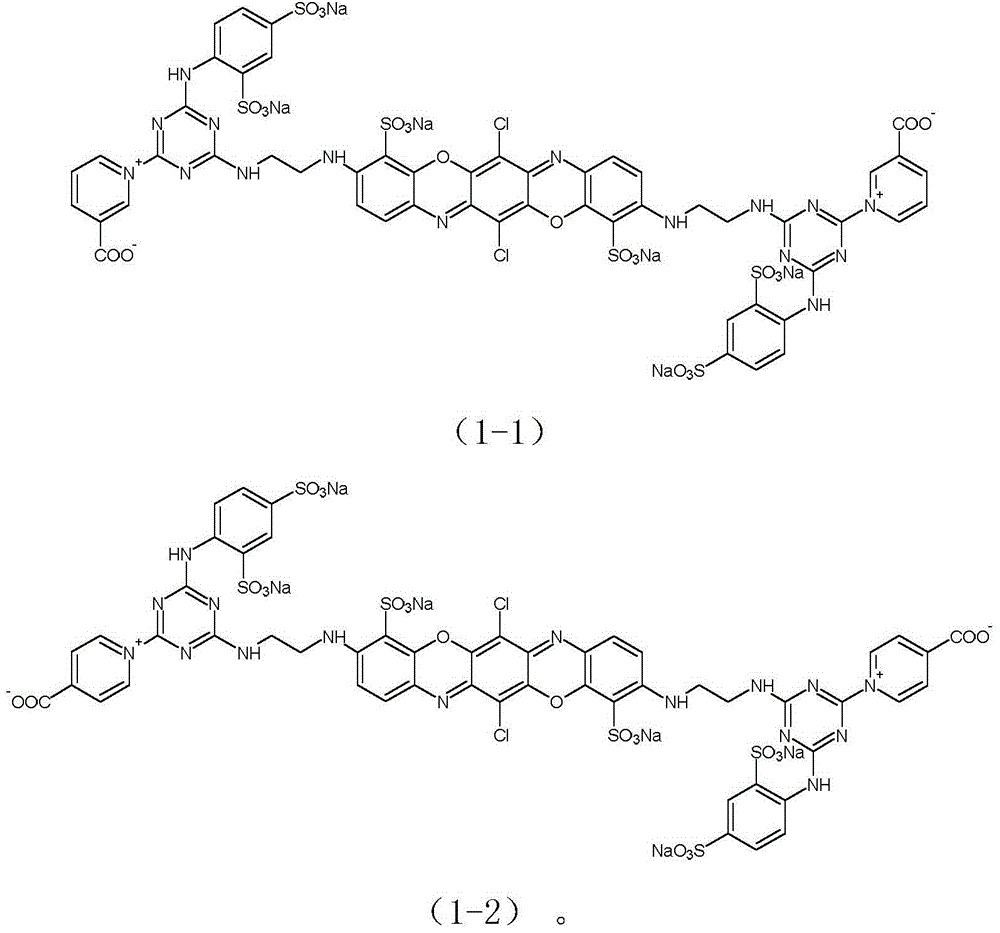
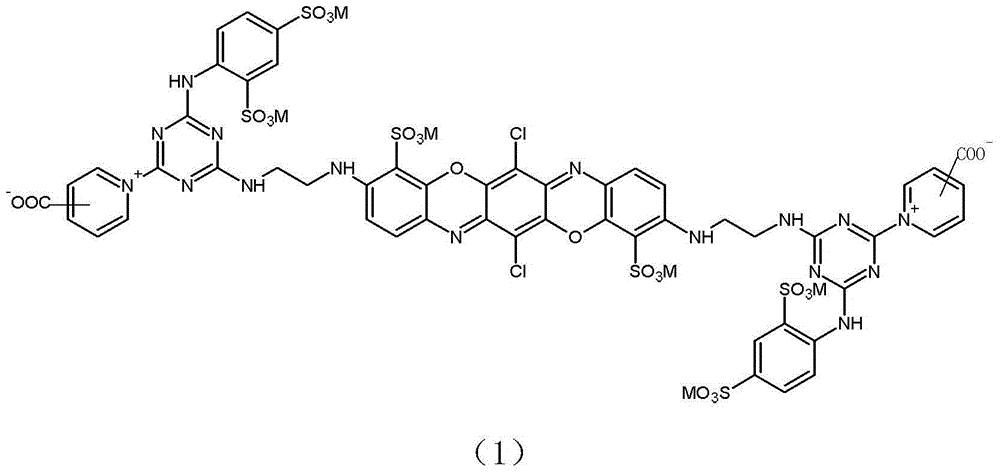
[0044] Example 1: Preparation of reactive brilliant blue of formula (1-1).
[0045] A, a shrinkage reaction
[0046] 8g of cyanuric chloride, 30g of water, 100g of ice, 1g of dispersant, and beating for 1 hour at 0-3°C, add 2.4-disulfonic acid aniline solution (pre-configuration: 10g of 2.4-disulfonic acid aniline + 100g of water, add soda ash dry powder Adjust pH=5-7 by 3g, stir and dissolve to clear), the feeding speed shall be controlled as pH≤5.0. After the addition, keep at 5-15℃ and react, then slowly add 5g baking soda to adjust pH=5-7, and the reaction will reach the end ( 9-10 hours).
[0047] B, dicondensation reaction
[0048] Once the end of the reduction is reached, add 25g of Reactive Blue 198 color base to the first reduction solution, adjust the pH to 7-9 with 3g of soda ash, after the addition, heat up to 30-50℃ for reaction, and use 4g of soda ash to keep the pH during the reaction =7-9, until the end of the reaction (reaction for 1-2 hours).
[0049] E. Triple shrinka
Embodiment 2
[0057] Example 2: Preparation of reactive brilliant blue of formula (1-2).
[0058] A, a shrinkage reaction
[0059] 8g of cyanuric chloride, 30g of water, 100g of ice, 1g of dispersant, and beating for 1 hour at 0-3°C, add 2.4-disulfonic acid aniline solution (pre-configuration: 10g of 2.4-disulfonic acid aniline + 100g of water, add soda ash dry powder Adjust pH=5-7 by 3g, stir and dissolve to clear), the feeding speed shall be controlled as pH≤5.0. After the addition, keep at 5-15℃ and react, then slowly add 5g baking soda to adjust pH=5-7, and the reaction will reach the end ( 9-10 hours).
[0060] B, dicondensation reaction
[0061] Once the end of the reduction is reached, add 25g of Reactive Blue 198 color base to the first reduction solution, adjust the pH to 7-9 with 3g of soda ash, after the addition, heat up to 30-50℃ for reaction, and use 4g of soda ash to keep the pH during the reaction =7-9, until the end of the reaction (reaction for 1-2 hours).
[0062] E. Triple shrinka
Embodiment 3
[0069] Example 3: Several formulas were selected according to the characteristics of superior application performance, etc., as shown in Table 3. The application properties of the reactive dyes prepared in the examples of the present invention are shown in Table 4 and Table 5 below.
[0070] table 3
[0071] Serial number
colour
Formula ratio
Formula (1-1)
Brilliant blue
Formula (1-1) 90g, Yuanming powder 10g
Formula (1-2)
Brilliant blue
Formula (1-2) 85g, Yuanming powder 15g
Formula (1-1) + Formula (1-2)
Brilliant blue
Formula (1-1) 45g, Formula (1-2) 45g, Yuanming powder 10g
[0072] Each reactive dye composition in Table 1 above is dry powder mixed in a mixing tank, or dissolved together and spray dried. There are other reactive dye compositions that are not listed one by one, but are included in the present invention.
[0073] Example 2: 100g cotton cloth, a low-alkali or alkali-free reactive brilliant blue dye obtained according to Example 1, 2% by weight of th
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap