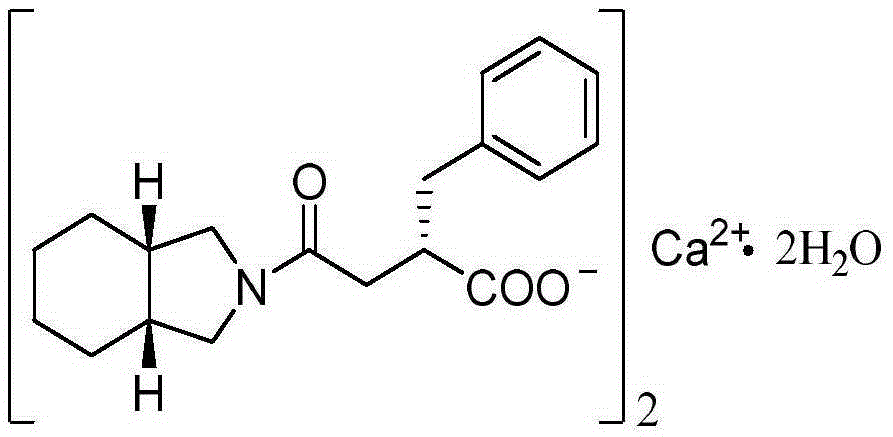
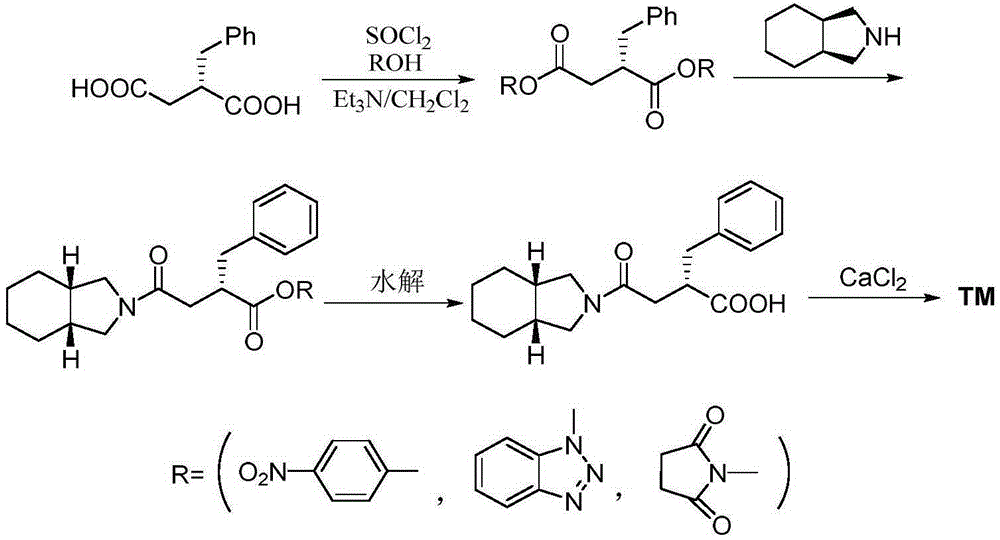
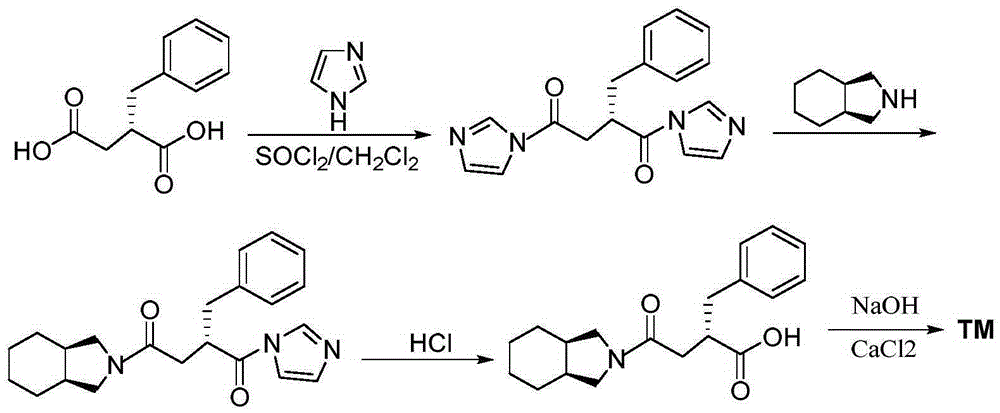
Novel mitiglinide calcium preparing method
A technology of mitiglinide calcium and mitiglinide acid, which is applied in the field of preparation of hypoglycemic drugs, can solve the problems of poor regioselectivity in amidation reaction steps, difficult product purification, and lack of industrialization, and achieve low production costs and high product quality. The effect of improved purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of (S)-2-benzylsuccinic anhydride
[0032] Add 30.40g (0.16mol) of (S)-2-benzylsuccinic acid, 20mL of acetic anhydride, and 60mL of toluene into a 100mL three-necked flask in sequence, and stir and reflux for 1 hour. After the reaction is complete, cool to crystallize, filter, and dry to obtain white flakes Crystals 25.39 g.
[0033] (2) Amidation of (S)-2-benzylsuccinic anhydride
[0034] Add 25.39 g (0.13 mol) of (S)-2-benzylsuccinic anhydride and 300 mL of dichloromethane into a 500 mL three-necked flask in sequence. Under the condition of -10~5°C, a dichloromethane solution (20 mL) of 17.54 g (0.14 mol) of cis-perhydroisoindole was slowly added dropwise, stirred for 4 hours, and the reaction was completed. The reaction solution was washed with 0.5 mol / L HCl solution (100 mL×2), and then washed with 100 mL saturated brine. The organic phase was dried overnight with 50 g of anhydrous sodium sulfate. After filtration, the filtrate was desolvated to o
Embodiment 2
[0042] (1) Preparation of (S)-2-benzylsuccinic anhydride
[0043] Add 30.40g (0.16mol) of (S)-2-benzylsuccinic acid, 20mL of acetic anhydride, and 50mL of toluene into a 100mL three-necked flask in sequence, and stir and reflux for 1 hour. After the reaction is completed, cool to crystallize, filter, and dry to obtain white flakes Crystal 26.12g.
[0044] (2) Amidation of (S)-2-benzylsuccinic anhydride
[0045] Add 26.12 g (0.14 mol) of (S)-2-benzylsuccinic anhydride and 300 mL of dichloromethane into a 500 mL three-necked flask in sequence. At -5-5°C, a dichloromethane solution of cis-perhydroisoindole (19.89 g, 0.16 mol) was slowly added dropwise, and the reaction was stirred for 5 h. After the reaction was completed, the reaction solution was washed with 0.5 mol / L HCl solution (100 mL×2), and then washed with 100 mL saturated brine. The organic phase was dried overnight with 50 g of anhydrous sodium sulfate. After filtration, the filtrate was desolvated to obtain 40.33 g
Embodiment 3
[0053] (1) Preparation of (S)-2-benzylsuccinic anhydride
[0054] Add 30.40g (0.16mol) of (S)-2-benzylsuccinic acid, 20mL of acetic anhydride, and 60mL of toluene to a 100mL three-necked flask, mix well, stir and reflux for 1 hour, cool to crystallize, filter, and dry to obtain white flakes Crystals 25.19 g.
[0055] (2) Amidation of (S)-2-benzylsuccinic anhydride
[0056] Add 25.19 g (0.13 mol) of (S)-2-benzylsuccinic anhydride and 300 mL of dichloromethane into a 500 mL three-necked flask in sequence. Under the condition of -10~0°C, a dichloromethane solution of cis-perhydroisoindole (18.54 g, 0.15 mol) was slowly added dropwise, and the reaction was stirred for 3 h. After the reaction was completed, the reaction solution was washed with 0.5 mol / L HCl solution (100 mL×2), and then washed with 100 mL saturated brine. The organic phase was dried overnight with 50 g of anhydrous sodium sulfate. After filtration, the filtrate was desolvated to obtain 41.89 g of a light yellow
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap