Processing method of adipose tissue, mesenchymal stem cell, single processed adipose cell, and extracellular matrix
A technology of adipose tissue and processing methods, applied in bone/connective tissue cells, tissue culture, animal cells, etc., can solve the problems of hidden safety hazards, low extraction efficiency, and long time-consuming, and achieve high safety, high efficiency, and easy operation short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0079] Example 1
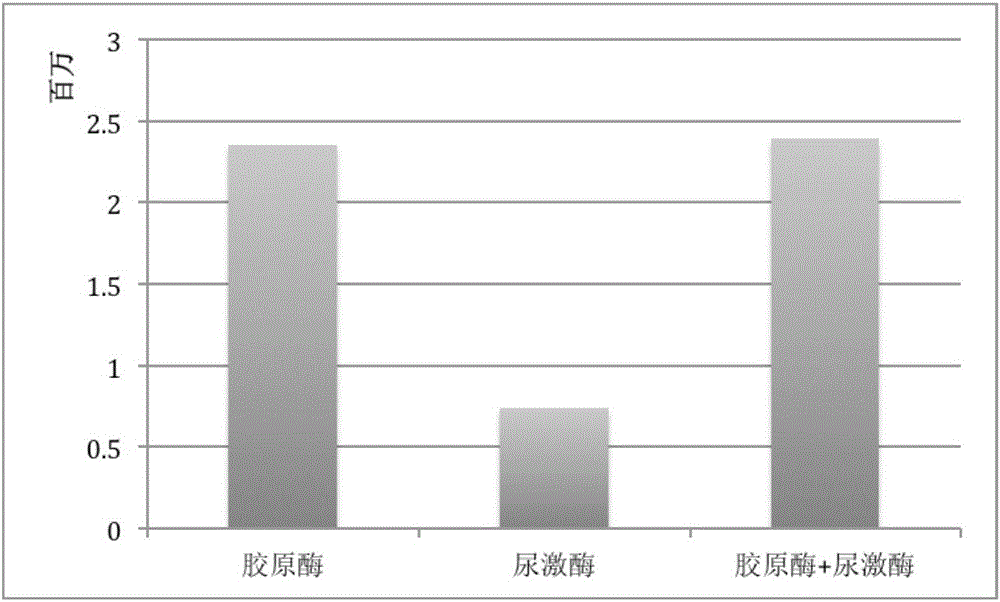
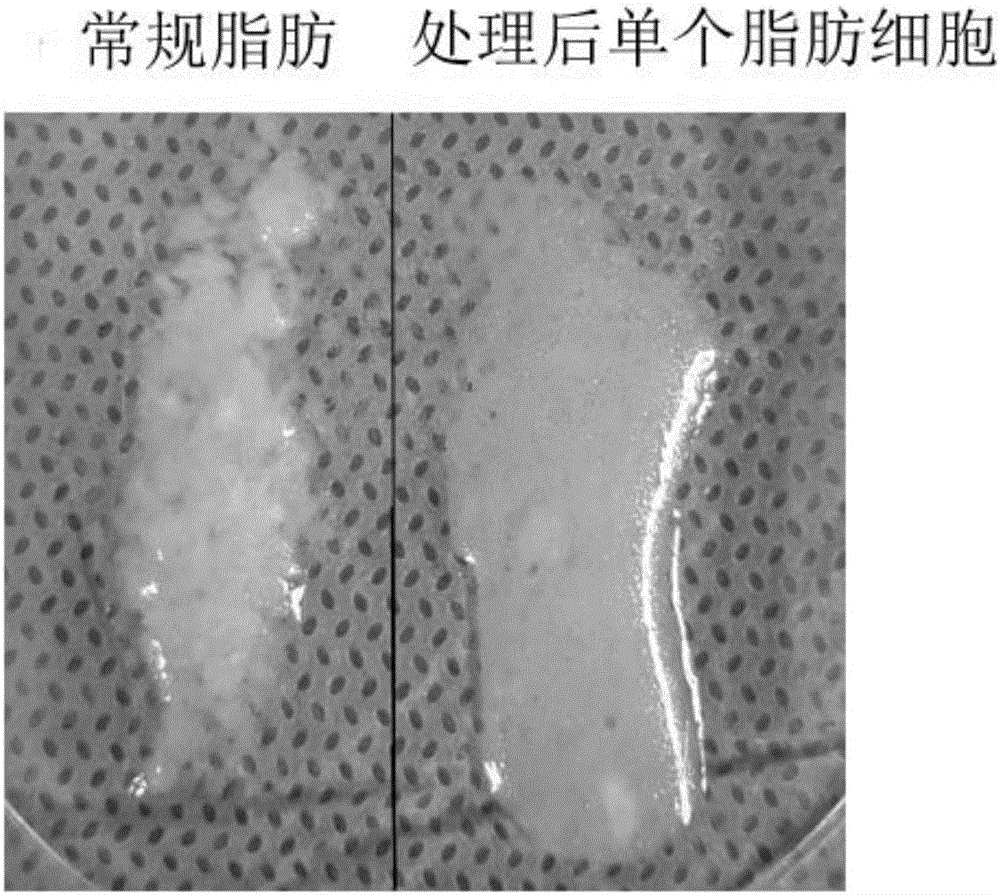
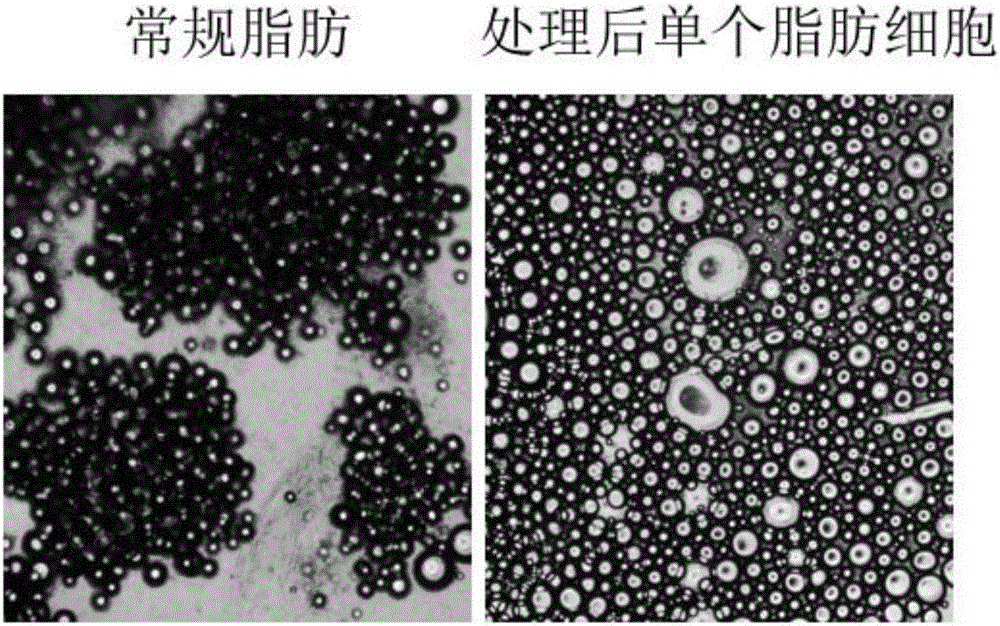
[0080] 2.4 g of adipose tissue was washed repeatedly with physiological saline to remove tumescent fluid and leukocytes in the adipose tissue. Then, the washed adipose tissue is fully minced with scissors until it is in the form of meat paste, and the particle size of the adipose tissue is between 1-2 mm, and placed in a sterile container. Add 300 units of collagenase for injection (certificate of Chinese medicine: H31022658, the same below), after shaking and digesting at 37°C for 30 min, add 5 mL of human albumin with a mass fraction of 20% (certified of Chinese medicine: S10980016, the same below) for neutralization injection With collagenase, the total number of cells was about 2.35 × 10 after filtration through a 70 μm cell sieve 6 indivual.
Example Embodiment
[0081] Example 2
[0082] 2.4 g of adipose tissue was washed repeatedly with physiological saline to remove tumescent fluid and leukocytes in the adipose tissue. Then, the washed adipose tissue is fully minced with scissors until it is in the form of meat paste, and the particle size of the adipose tissue is between 1-2 mm, and placed in a sterile container. Add 300 units of collagenase for injection (H31022658) and 125,000 units of urokinase (H44020645), shake and digest at 37°C for 30 minutes, then add 10 mL of human serum albumin with a mass fraction of 20% for neutralization Collagenase and urokinase for injection, filtered through a 70μm cell sieve, the total number of cells was about 2.39×10 6 indivual.
Example Embodiment
[0083] Example 3
[0084]2.4 g of adipose tissue was repeatedly washed with physiological saline to remove tumescent fluid and leukocytes in the adipose tissue. Then, use scissors to fully cut into pieces, and after the washed adipose tissue is in the shape of meat paste, and the particle size of the adipose tissue is between 1-1.5 mm, it is placed in a sterile container. 300 units of collagenase for injection were added, and after 30 min of shaking at 37°C, 5 mL of human serum albumin with a mass fraction of 20% was added to neutralize the collagenase for injection. After centrifugation at 2400 r / min for 5 min, the cell pellet was collected. Add physiological saline to resuspend the cell pellet and filter it with a 70 μm pore size cell sieve to obtain a single cell suspension. Then centrifuge at 1200r / min to collect the precipitate, remove the supernatant, remove the collagenase for injection and human albumin, and obtain a total number of cells of about 2.16×10 6 indivua
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap