Proline protease mutant and application thereof
A proline protease and mutant technology, which is applied in the field of proline protease mutant application, can solve the problem of low homology in the primary structure, and achieve the effect of reducing turbidity and turbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The acquisition of embodiment 1 proline protease gene
[0023] The genome of an Aspergillus niger (Aspergillus niger) Su2-1 preserved in our laboratory was extracted. PCR amplification was performed using primer F and primer R described below. The reaction conditions were: denaturation at 94°C for 5 minutes; then denaturation at 94°C for 30 s, renaturation at 56°C for 30 s, extension at 72°C for 120 s, and after 30 cycles, incubation at 72°C for 10 min. The amplified product was subjected to agarose electrophoresis, and the result showed that the size of the amplified gene fragment was 2100bp.
[0024] Primer F: ATGCGTTCCTTTCTCCGCTGTCG
[0025] Primer R: TCAAGCATAATACTCCTCCACC
[0026] The obtained gene fragments were sent to Shanghai Sangon Bioengineering Co., Ltd. for sequencing. Sequencing results show that the nucleotide sequence of the gene fragment is SEQ ID NO: 2, and the encoded amino acid sequence is SEQ ID NO: 1. Through NCBIBLAST comparison, it was found th
Embodiment 2
[0027] The acquisition of embodiment 2 proline protease mutants
[0028] In order to improve the heat resistance of the above-mentioned proline protease F1, the applicant screened a large number of mutations of the enzyme by directed evolution technology.
[0029] Using the F1 gene as a template, PCR amplification was carried out with the GeneMorph II Random Mutation PCR Kit (Stratagene) using primers F and primer R, and the PCR product was recovered from the gel, digested with EcoRI and NotI, and then digested with pET21a vector after the same enzyme digestion Connect, transform into Escherichia coli BL21(DE3), spread on LB+Amp plate, culture upside down at 37°C, after the transformants appear, pick them one by one into a 96-well plate with a toothpick, add 150ul containing 0.1mM IPTG to each well LB+Amp medium, cultured at 37°C and 220rpm for about 6 hours, centrifuged to discard the supernatant, resuspended the bacteria with pH 5.5 buffer, repeatedly freeze-thawed to break the
Embodiment 3
[0034] Embodiment 3 Construction of recombinant expression vector
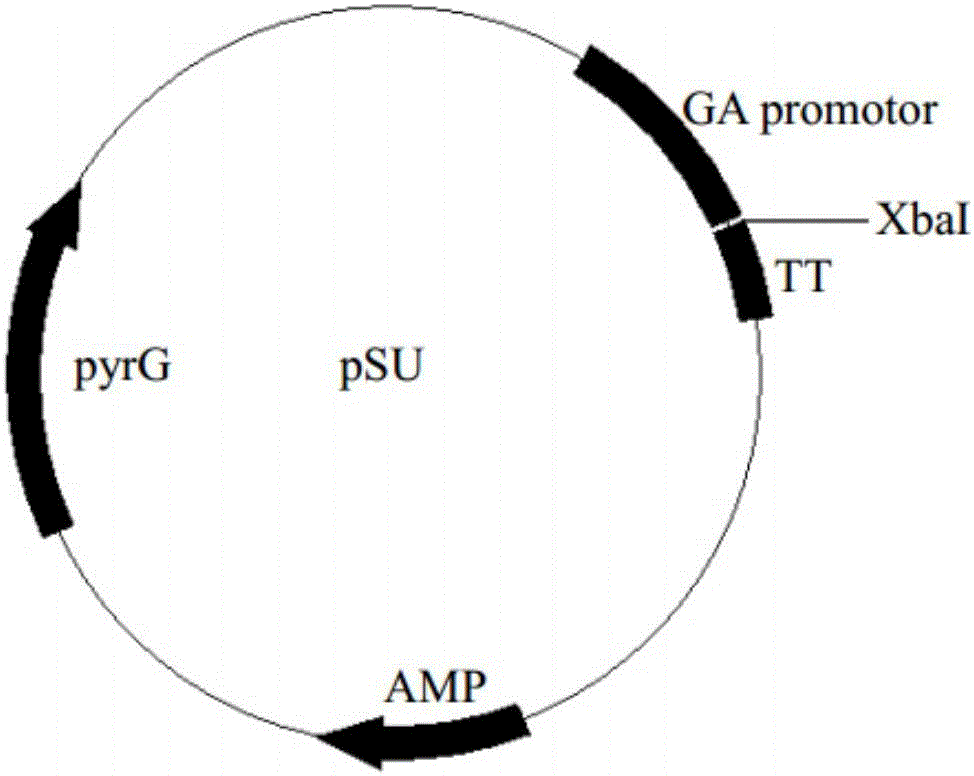
[0035] The proline protease F1 and its mutant F2 genes were connected to the expression vector pSU ( figure 1 ), the expression vectors pSU-F1 and pSU-F2 were constructed.
[0036]Protoplast preparation: Inoculate the host fungus Aspergillus niger Su2-1 on a PDA+U plate and culture at 30°C for 5-7d. Bacterial blocks with a size of 2cm×2cm were cut out, inoculated into 100ml liquid PDA+U medium, and cultured at 30°C for 24h to grow mycelia for transformation. After filtering the grown mycelia, resuspend with 20ml of 1.2M magnesium sulfate solution, and add 0.2g of lysozyme. Cultivate at 30°C and 100rpm for 2-3h. The lysed hyphae were filtered with two layers of lens paper, and centrifuged at 3000rpm for 10min to obtain protoplasts.
[0037] Transformation: the protoplasts were washed twice with 1.2M sorbitol solution, and then resuspended with an appropriate amount of sorbitol solution to make the protoplast c
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap