Near-infrared fluorescence probe for rated quantitative determination on endogenous hydrogen peroxide and preparation method and application of near-infrared fluorescent probe
A quantitative detection technology of hydrogen peroxide, applied in the field of chemical analysis and detection, can solve the problems of not revealing the effect of hydrogen peroxide well, low probe selectivity, large interference, etc., and achieve high selectivity and high purity , the effect of reducing interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
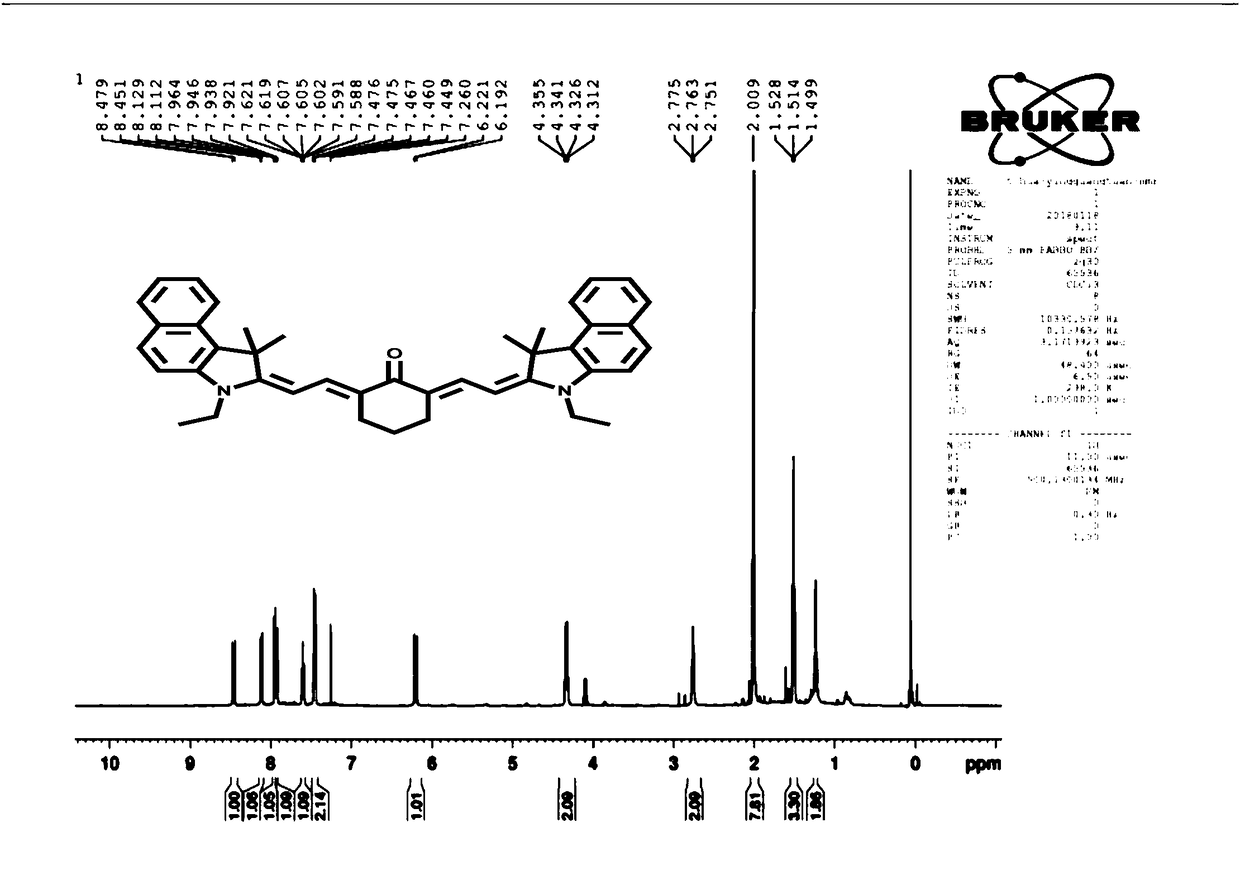
Embodiment 1
[0043] Preparation of organic compound of formula I based on cyanine:
[0044] (1) Ethyl group on phenacindole: Dissolve benzoindole (24g) and ethyl iodide (18g) in 50mL of anhydrous acetonitrile at 80°C and reflux for 12h to obtain the purple product a;
[0045] (a);
[0046] (2) Take 40mL of anhydrous DMF and 40mL of anhydrous dichloromethane and mix them evenly to obtain a mixed solvent. Stir the mixed solvent at -10°C for 20 minutes, and add 37mL of POCl dropwise during the stirring process 3 Mix the solution with 35mL of anhydrous dichloromethane, then add 10g of cyclohexanone, stop cooling for 2-3h, raise the temperature to 45°C and heat for 3h, pour the yellow liquid obtained from the reaction into ice while stirring, overnight, and suction filter Vacuum drying gives yellow solid b;
[0047] (b);
[0048](3) Weigh 1 g of b and 6 g of a, dissolve b and a in a mixed solution of 210 mL of toluene and 90 mL of n-butanol, heat to reflux at 80°C, and purify by silica ge
Embodiment 2
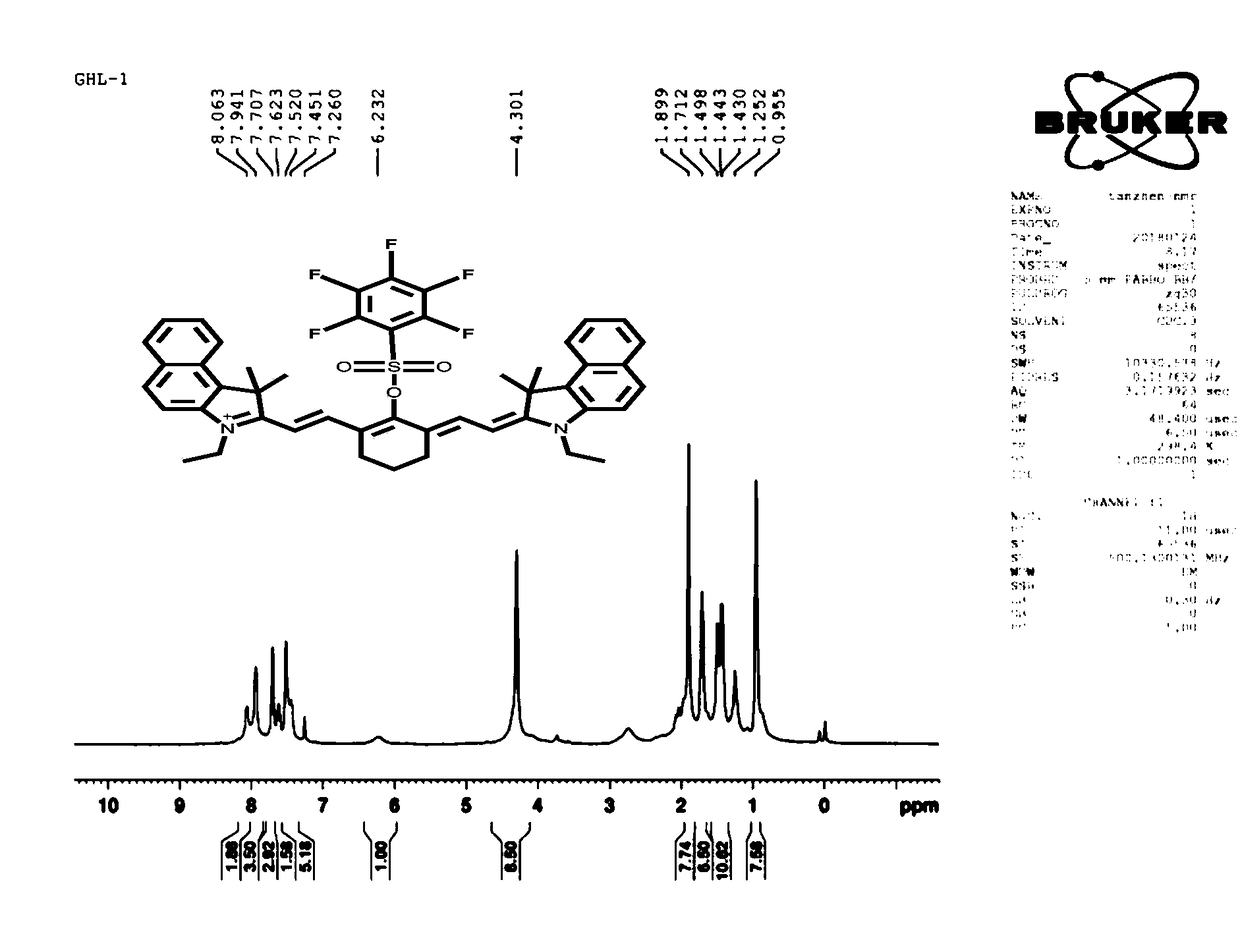
[0055] The prepared compound of formula I was used as a probe to detect hydrogen peroxide in cells, tissues and organs to simulate physiological conditions. The following experiments were carried out at pH = 7.4 (HEPES buffer solution, concentration 40 mM) , the concentration of the probe was 10 μM.
[0056] The ultraviolet response of the compound of formula I prepared above to MAO:
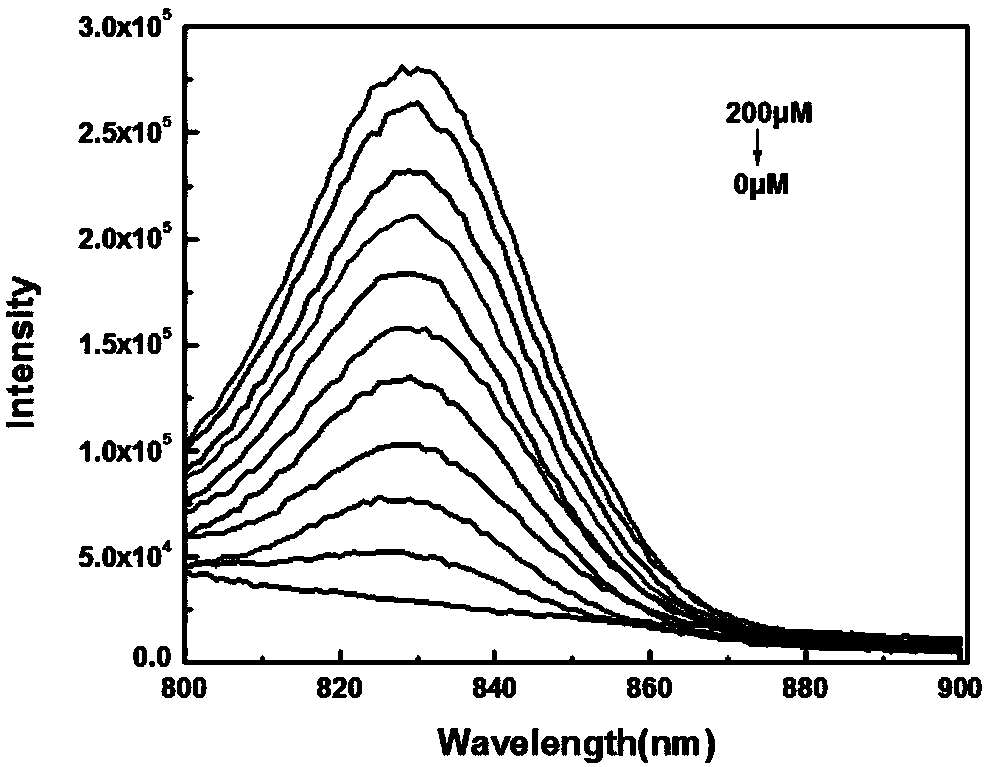
[0057] pH was controlled with HEPES buffer solution. Add 10 μM compound formula 1 to a 10 mL colorimetric tube, then add 40 mM HEPES, then add hydrogen peroxide, dilute with ultrapure water and set the volume to 0-200 μM, shake the solution, and equilibrate for 10 minutes, then add the above working solution The UV absorption spectrum was measured in a cuvette. The changes of ultraviolet absorption spectrum before and after detecting hydrogen peroxide are as follows: image 3 and 4 As shown, the changes in ultraviolet absorption of fluorescent probes before and after hydrogen peroxide detection
Embodiment 3
[0059] Embodiment 3 Formula I compound is to the selectivity of hydrogen peroxide
[0060] pH was controlled with HEPES buffer solution. Take multiple 10 ml colorimetric tubes, and add 10 μM compound formula 1 to each 10 ml colorimetric tube, then add 40 mM HEPES buffer solution with pH 7.4, and then add such as Image 6 As shown, the analytes are as follows: H 2 0 2 , t-BuO, Glu, TBHP, O2 - ,NO, . OH,OCl - , Cys, His, GSH. Finally, dilute to 10 ml with ultrapure water. Shake the solution evenly, and after equilibrating at 25°C for 10 min, pour the working solution in each colorimetric tube into a fluorescent dish to measure the fluorescence spectrum. Compound of formula one to hydrogen peroxide - selectivity such as Image 6 shown. And by Image 6 It can be seen that the compound of formula one has good selectivity to hydrogen peroxide, and specifically recognizes hydrogen peroxide. Compared with hydrogen peroxide probes in the prior art, the probe prepared by the pr
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap