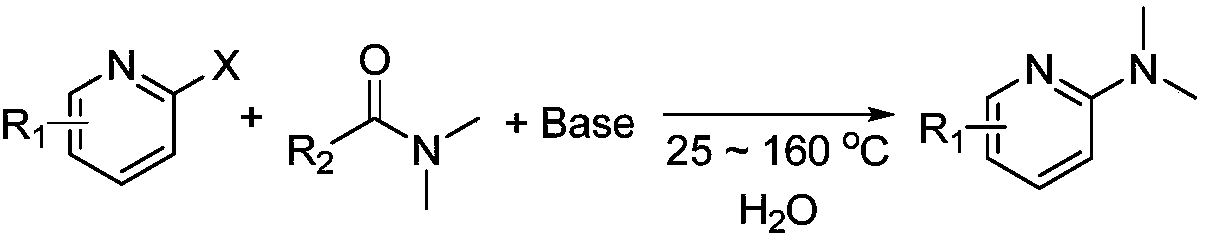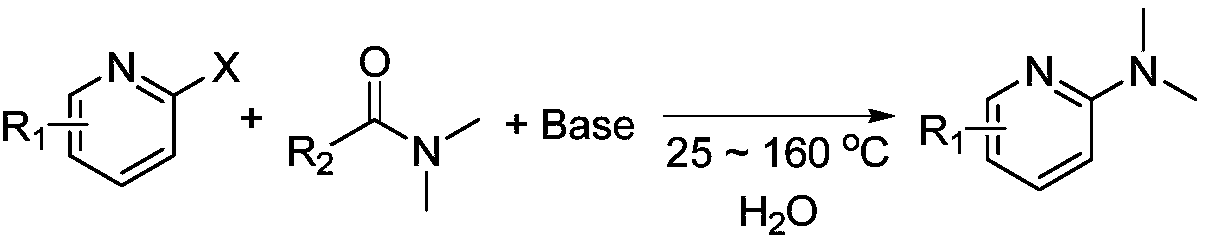Novel green and practical synthesis method of N,N-dimethyl pyridine compound
A kind of technology of lutidine and compound, applied in the field of synthesis of N,N-lutidine compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 2,5-dibromopyridine (1 mmol), DMF (1 mmol), inorganic base potassium tert-butoxide (sodium) or potassium trimethylsiliconate (sodium) (1 mmol), and 2 mL of water into a 10 mL sealed tube in sequence, Heated and stirred in an oil bath at 100°C for 6 hours, and followed the reaction process according to TLC. After the reaction was completed, an equivalent amount of o-phthalylene dimethyl ether was added to the crude product as an internal standard to determine the exact yield of the product by GC and GC-MS. According to GC and GC-MS, when DMSO is used as the reaction solvent, the inorganic base potassium tert-butoxide (sodium) or potassium trimethylsiliconate (sodium) is used as the catalyst, and the yield of the product is 42%, 59%, 67%, respectively. %, 60%. Product NMR data: 1 H NMR (400MHz, CDCl 3 ):8.15(s,1H),7.47(d,J=8Hz,1H),6.39(d,J=8Hz,1H),3.04(s,6H).
Embodiment 2
[0021] Add 2,5-dibromopyridine (1.0mmol), DMF (1mmol), sodium tert-butoxide (0.5-4.0mmol), and 2mL water into a 10mL sealed tube in turn, heat and stir in an oil bath at 140°C for 6 hours, and use two Chloromethane quenching reaction, when the reaction is over, adding internal standard to the crude product can determine the yield of the product by GC and GC-MS, when sodium tert-butoxide is 0.5mmol, 1.0mmol, 2.0mmol, 3.0mmol, At 4.0 mmol, the isolated yields of the products were 55%, 66%, 72%, 80%, and 82%, respectively.
Embodiment 3
[0023] Add 2,5-dibromopyridine (1.0mmol), DMF (1mmol), sodium tert-butoxide (3.0mmol), and 2mL water into a 10mL sealed tube in turn, heat and stir in an oil bath at 25-140°C for 6 hours, and use two Chloromethane quenched the reaction. When the reaction was over, the crude product was added with an internal standard to determine the yield of the product by GC and GC-MS. When the temperature was 25°C, 80°C, 100°C, and 140°C, the yield of the product The isolated yields were 0%, 36%, 59%, and 80%, respectively.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap