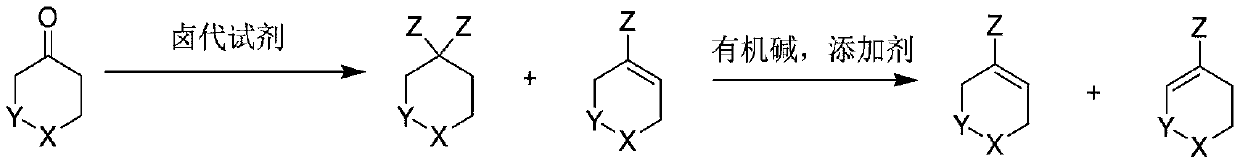
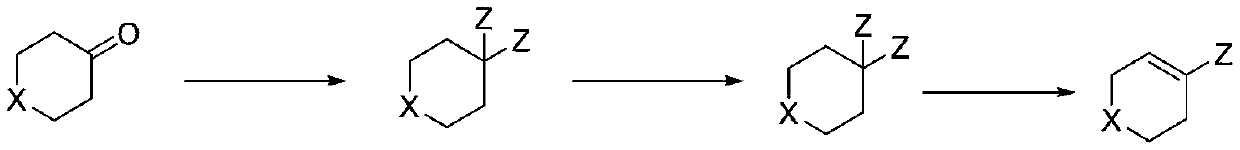Preparation method of heteroatom-containing cyclohexene halide
A cyclohexene and heteroatom technology, applied in the field of fine chemical intermediate synthesis, can solve the problems of harsh reaction conditions, affecting industrial production, and difficult to deal with three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0027] Example 1:
[0028] Under the protection of nitrogen, add tetrahydropyran-4-one (10.0g, 0.1mol) and 50.1g 1,2-dichloroethane to a 250mL three-necked reaction flask equipped with a condenser and a constant pressure low liquid funnel. Under stirring, the temperature in an ice-salt bath was cooled to 5-10°C and thionyl chloride (16.9g, 0.15mol) was added dropwise. After the dropwise addition was completed, it was heated to reflux for 5.0 hours. Send samples to detect that the residual raw material is less than 0.5%, GC and GC-MS did not detect tetrahydropyran-4-enyl chloride, concentrated to remove excess thionyl chloride, concentrated solution (GC: 97.8%, GC-MS :154.0) No purification is required to proceed directly to the next reaction.
[0029] Put DBU (18.3g, 0.12mol), NaNH into the reaction flask containing the concentrate in the previous step 2 (7.8g, 0.2mol) and 50.2g 1,2-dichloroethane. After feeding, heat to 80°C and stir for 3.0 hours. Send samples to detect that the
Example Embodiment
[0030] Example 2:
[0031] Under the protection of nitrogen, add tetrahydrothiopyran-4-one (11.6g, 0.1mol) and 58.1g 1,4-dioxane to a 250mL three-necked reaction flask equipped with a condenser and a constant pressure low liquid funnel. Under stirring, the temperature in an ice-salt bath was cooled to 5-10°C and oxalyl chloride (19.1g, 0.15mol) was added dropwise. After the dropwise addition was completed, the mixture was heated under reflux and stirred for 6.0 hours. The sample was sent to detect that the residue of the raw material was less than 0.5%, and the tetrahydrothiopyran-4-enyl chloride was not detected by GC and GC-MS. The concentration was started to remove the excess oxalyl chloride. The concentrated solution (GC: gem dichloride 95.5%, two Oxane 4.1%) does not need to be purified directly to the next step.
[0032] Put TMG (17.3g, 0.15mol), LiNH into the reaction flask containing the concentrate in the previous step 2 (4.6g, 0.2mol) and 58.1g 1,4-dioxane. After feeding
Example Embodiment
[0033] Example 3:
[0034] Under the protection of nitrogen, add N-benzylcyclohexyl-4-one (18.9g, 0.1mol) and triethylamine (11.2) to a 250mL clean and anhydrous three-necked reaction flask equipped with a condenser and a constant-pressure low-liquid funnel. g, 0.11 mol) and 94.6 g of a mixture of acetonitrile and sulfolane (mass ratio of 5:1), replaced with nitrogen three times, heated to 30-40° C. with stirring, and added phosphorus pentachloride (24.9 g, 0.12 mol) in batches. After the addition is complete, heat and stir at 80°C for 5.0 hours. The sample is sent to GC to detect that the residual raw material is less than 0.5%, and the product contains alkenyl chloride and geminal dichloride (confirmed by molecular weight in GC-MS), and the proportions of the two are 81% and 19% (only the two peaks are retained). The ratio of the hydrogen spectrum detection conversion between the two is 78:22. The reaction solution does not need to be purified directly to the next step. (Compar
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap