Liposomal enhanced intra-peritoneal chemotherapy
A technology of liposome preparation and liposome composition, applied in the field of treatment of ovarian tumors and peritoneal tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
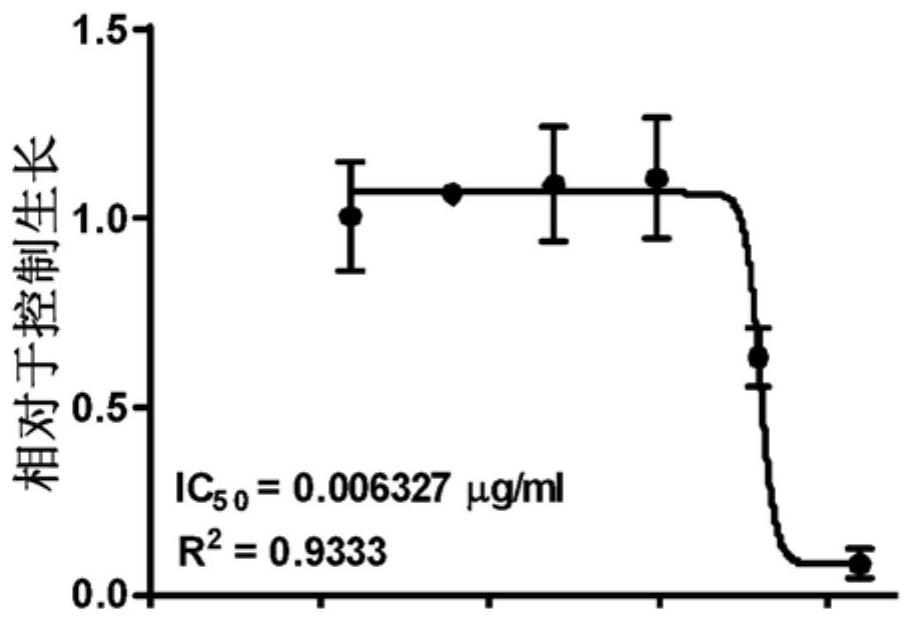
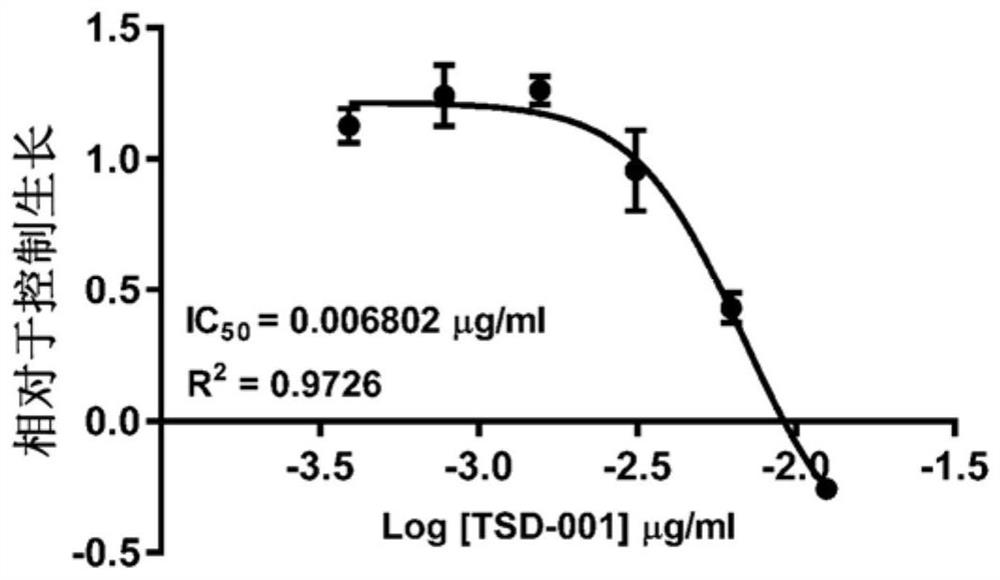
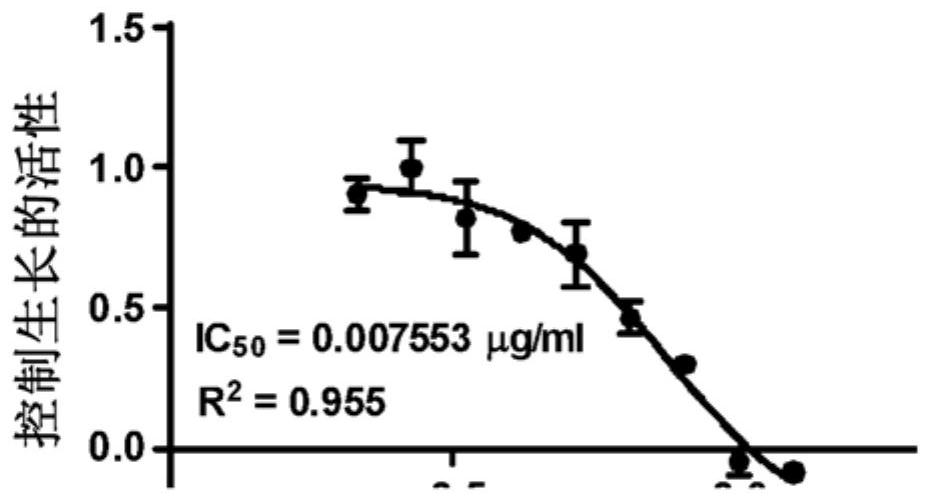
[0057] Example 1. TSD-001 treatment of paclitaxel IC in human ovarian cancer cells 50 Determination. The inhibitory concentration of paclitaxel for Ovcar3-RFP-based ovarian cancer cells (IC) was determined by sulfonyl rausein B (SRB) analysis. 50 The TSD-001 liposome preparation (paclitaxel: DMPC: DMPG = 1: 1.43: 0.567) was treated with Ovcar3-RFP human ovarian cancer cells. For analysis, the lyophilized TSD-001 was re-formulated with sterile injection grade water, and the concentration was 6 mg / ml, then in RPMI cell media (RPMI-1640, Corning, Ltd. containing L-glutamine) in RPMI cell culture medium. Continuous dilution. Three independent dose curves were carried out. In two analyzes, the series dilution concentration of TSD-001 is between 0.05 μg / ml to 0.391 ng / ml, in the third analysis, the series dilution concentration of TSD-001 is at 0.0128 μg / ml to 2.147. Ng / ml is between.
[0058] Also prepared Paclitaxel formulation (binding paclitaxel) and a series of di
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap