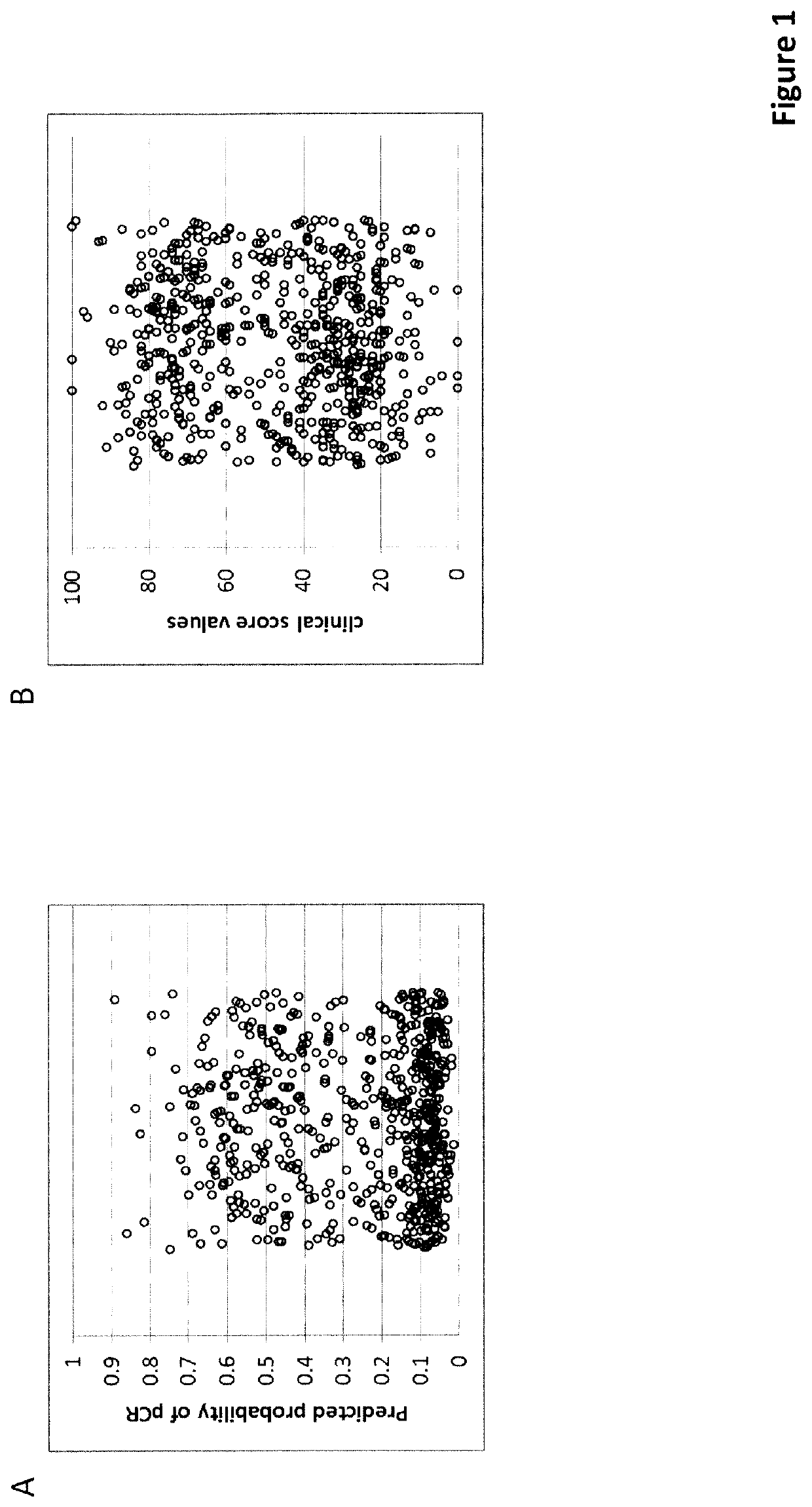
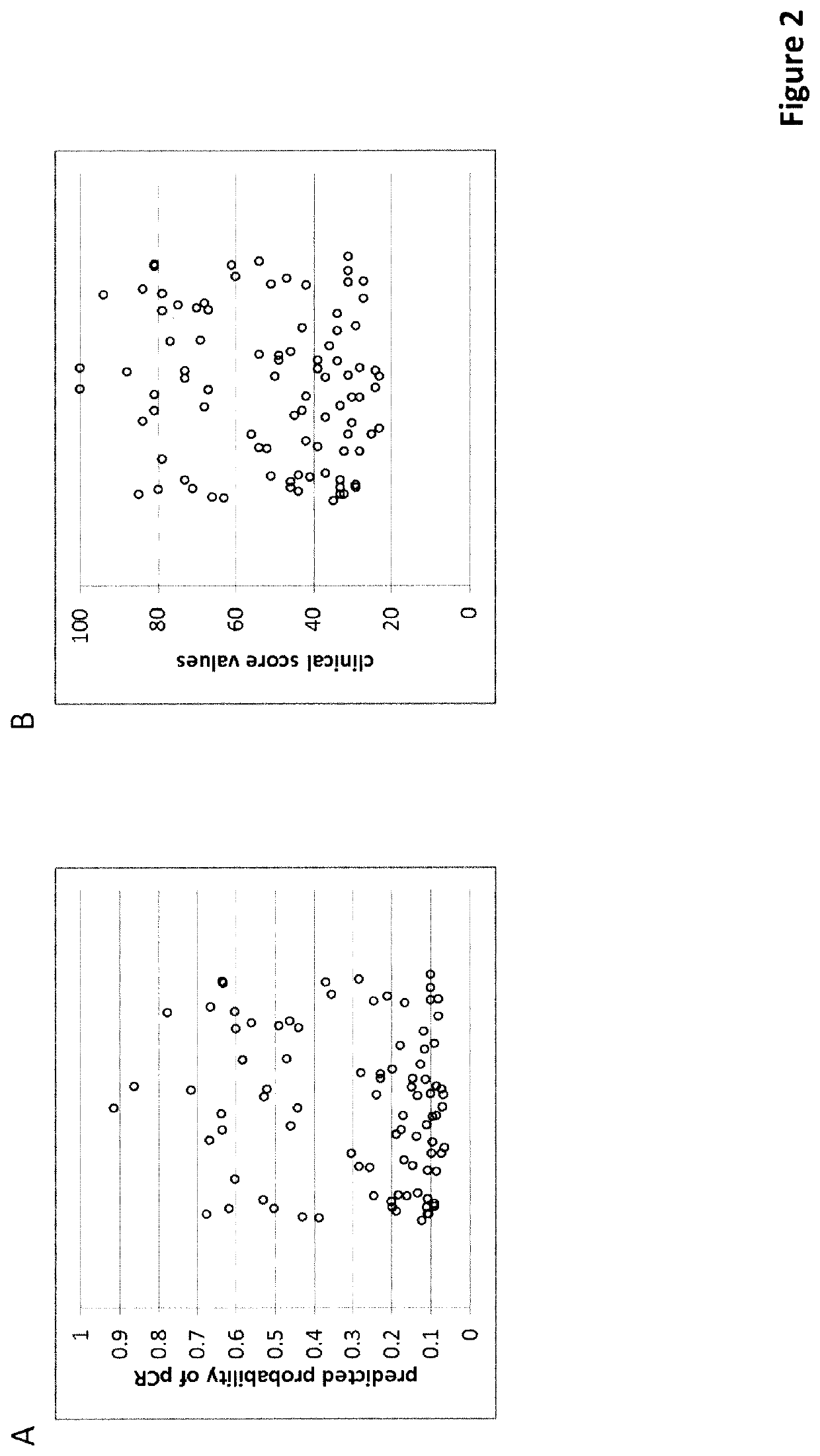
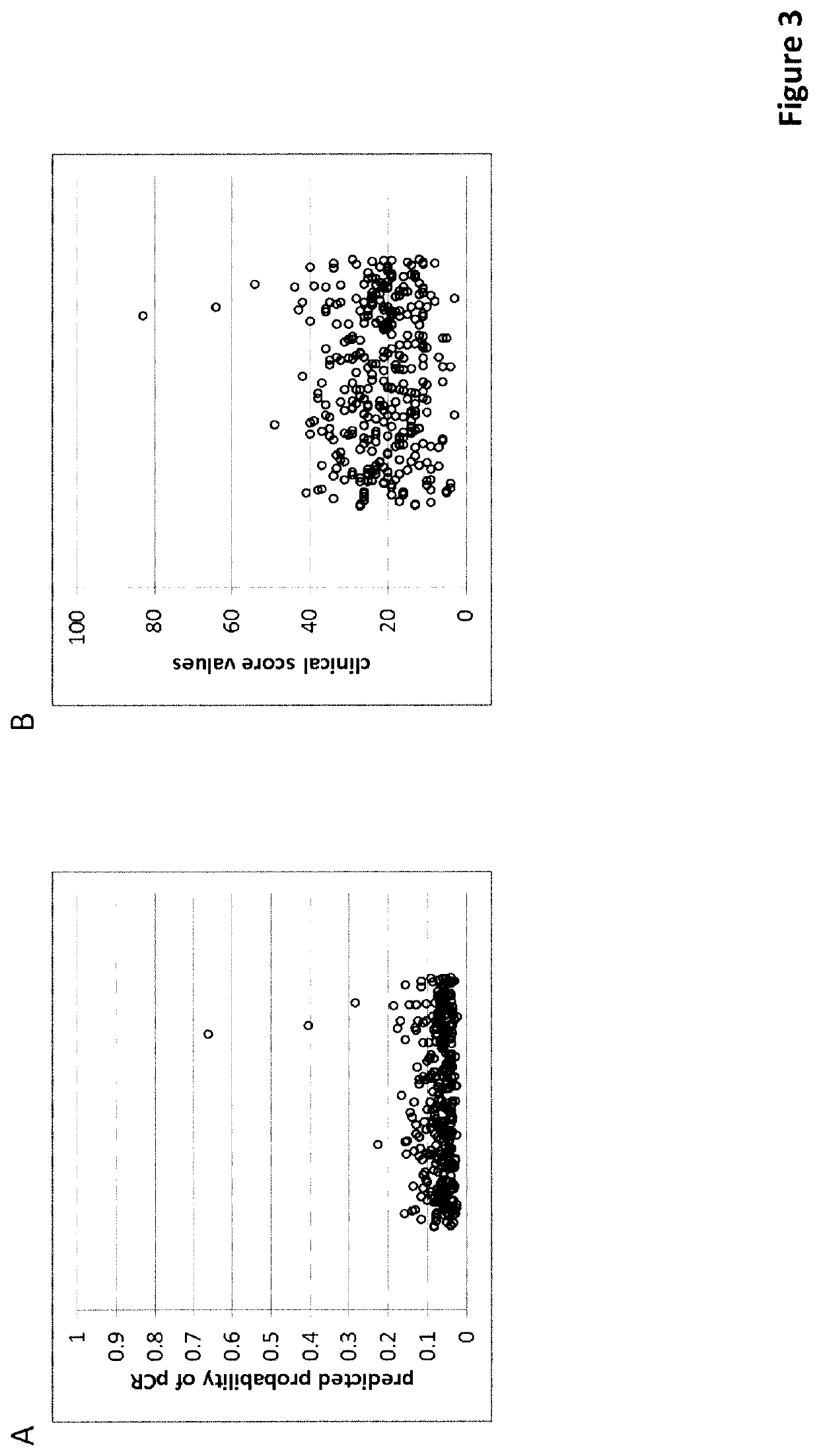
Predictive and Prognostic Methods in Breast Cancer
a breast cancer and prognostic method technology, applied in the field of breast cancer prediction and prognostic methods, can solve the problems of no method widely accepted as standard and applied routinely, and achieve the effect of increasing the probability of pcr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Total RNA from FFPE Samples Using the RNXtract© Protocol
[0387]Fixation of tumor tissue with formalin and subsequent embedding in paraffin is a standard method in clinical pathology and allows long-term archiving of samples. Because of chemical modifications of nucleic acids in FFPE samples, special protocols are necessary to extract amplifiable nucleic acids. Three steps are required for this: (1) removal of the paraffin, (2) lysis of the tissue and release of RNA (de-modification of nucleic acids if required), (3) purification of RNA by several washing steps.
[0388]The RNXtract® kit (BioNTech Diagnostics GmbH, Mainz, Germany) allows purification without organic solvents, which can be conducted in a single reaction vessel.
[0389]In the first step, the paraffin contained in the FFPE sections is liquefied in an optimized lysis buffer. Subsequent addition of proteinase K leads to lysis of the tissue and release of cellular nucleic acids (RNA and DNA). The RNA is bound to magnetic particles,
example 2
the Gene Expression Level of the Biomarkers Using the MammaTyper® Kit
[0390]The MammaTyper® kit (BioNTech Diagnostics GmbH, Mainz, Germany) allows the determination of the level of expression of selected biomarkers at the mRNA level by means of reverse transcription quantitative PCR (RT-qPCR).
[0391]To determine the expression level of a biomarker at transcript level by PCR, RNA has first to be transcribed into complementary DNA (cDNA) via the enzyme reverse transcriptase (so-called first strand synthesis). The marker-specific cDNA is then amplified by a DNA polymerase and amplification is detected in the PCR in real time using fluorescently labeled hydrolysis probes. The RT-qPCR takes place as a one-step reaction in the MammaTyper® assay, i.e., reverse transcription of the RNA and subsequent PCR of the DNA occur consecutively in the same reaction mixture. In addition to the enzymes (reverse transcriptase and DNA polymerase), the enzyme mix contains dNTPs as well as salts and PCR additiv
example 3
of an Unscaled Score (Score 1)
[0399]The unscaled score was trained on a set of routine FFPE biopsies from patients who received neo-adjuvant chemotherapy at the University Clinics of Erlangen (Germany) between 2000 and 2015. After selecting samples with sufficient tissue available for sectioning, a minimum of 20% tumor cell content and sufficient RNA for a MammaTyper® test (valid result) a total of 598 samples were included into the study. The MammaTyper® test (BioNTech Diagnostics GmbH, Mainz, Germany) was performed according to the manufacturer's instructions on RNA extracted from a 10 μm curl from each sample using the nucleic acid isolation kit RNXtract® according to the manufacturer's instructions. The MammaTyper® measurements were performed on a LightCycler® 480 II (Roche Diagnostics). The samples from the cohort also fulfilled these inclusion / exclusion criteria.
[0400]Inclusion Criteria[0401]Female patients of the gynecology department of the University Clinics of Erlangen (Ger
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap