Preparation method of isoindolinone pigment
A technology of isoindolinone and pigments, which is applied in the field of preparation of isoindolinone pigments, can solve the problems of poor weather resistance, heavy pollution and high cost, and achieve the effects of cheap raw materials, high yield and high tinting strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
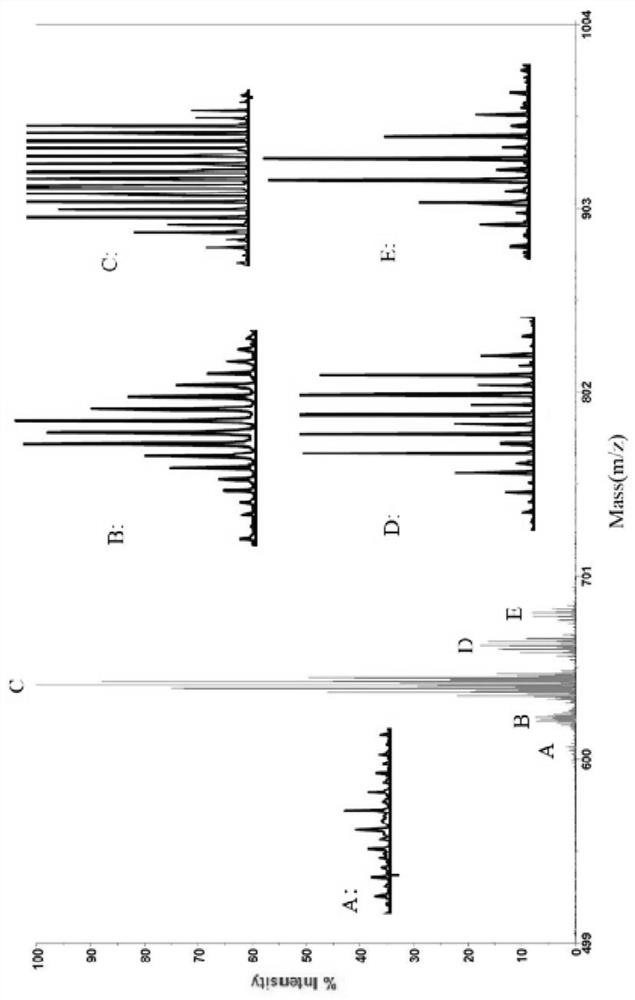
Image
Examples
Example Embodiment
[0044] Example 1
[0045] Tetrachlorochemisodiphenimic anhydride was 250g, phosphorus phosphorus 182 g, and phosphorus 160g of extol. The chlorinated product was introduced in advance to the reactor of 245 g of ammonia water, and the reactor of 3 g of sodium hydroxide was 3 h. Next, the reaction was reacted for 2 hours at a pressure vessel at 120 ° C. Then cool the temperature, separated, 236.5 g of intermediate TCCBM, ie (1), HPLC detects 99.2%; excess of dimethyl carbonate recovery.
[0046] The 20 g of intermediate TCCBM was added to 88 mL of methanol, and the organic base was 11.5 g of stirring for half an hour, then the 3.6 g of phenylenediamine dissolved in advance was 2 hours at normal temperature, and the temperature was continued to 50 ° C for 1 hour, then transferred to The reaction was reacted in 80 ml of water for 0.5 hours, and the pH is adjusted to 5.5 with a small amount of acetic acid / methicate, cooling filtration, washing, the resulting filter cake is pulped into
Example Embodiment
[0051] Example 2
[0052] The intermediate TCCBM obtained by 20 g of Example 1 was added to 100 mL of methanol, and the organic base was 12.1 g and stirred for half an hour, and then the 3.55 g of phenylenediamine dissolved in advance was 1 hour at 45 ° C, then warmed to 70 ° C. 1.5-2 hours, then transferred to 80 ml of water to react for 1 hour, adjusting the pH to 6.0 with a small amount of formic acid / hydrochloric acid, cooling filtration, washing, pulling the resulting filter cake, 0.5 g of alkyl naphthalenesulfonic acid, at 50-80 The reaction was reacted for 2 hours, then filtered, washed, dried.
Example Embodiment
[0053] Example 3
[0054] The intermediate TcCBM obtained by 20 g of Example 1 was added to 90 mL of methanol, and the organic base was 12.0 g of stirring for half an hour, then 4.05 g 2,6-dicharobenzene is reacted at room temperature for 0.5 hours, then warmed to 45 At ° C, the reaction was 1 hour, and finally heated to reflux continued to react for 1-2 hours, and finally transferred to 75 ml of water for 1 hour, and the pH is adjusted to 5.5 with a small amount of acetic acid / sulfate, and the filtered, washed, and the resulting filter cake was pulled. 0.4 g of quaternary ammonium salts were added, and the reaction was reacted at 30-60 ° C for 2 hours, then filtered, washed, dried, and finally green yellow pigment PY 109, 21.9 g.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap