Apparatus for purification of industrial wastewater with thin film fixed bed TiO2 photocatalyst
a technology of industrial wastewater and apparatus, applied in physical/chemical process catalysts, other chemical processes, separation processes, etc., can solve the problems of limiting practical applications and high water contamination of several lakes, and achieve the effect of easy recycling and easy removal of recalcitrants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
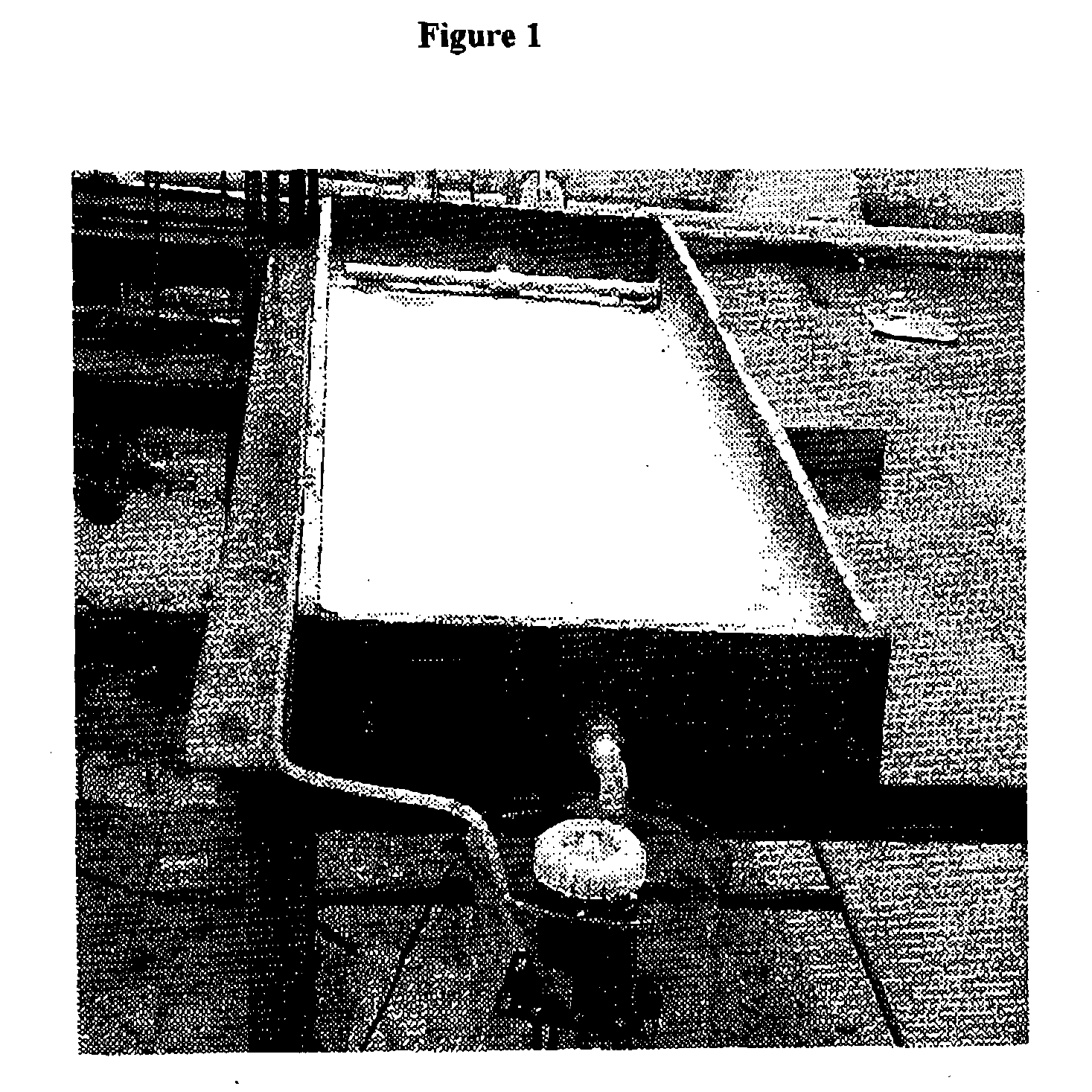
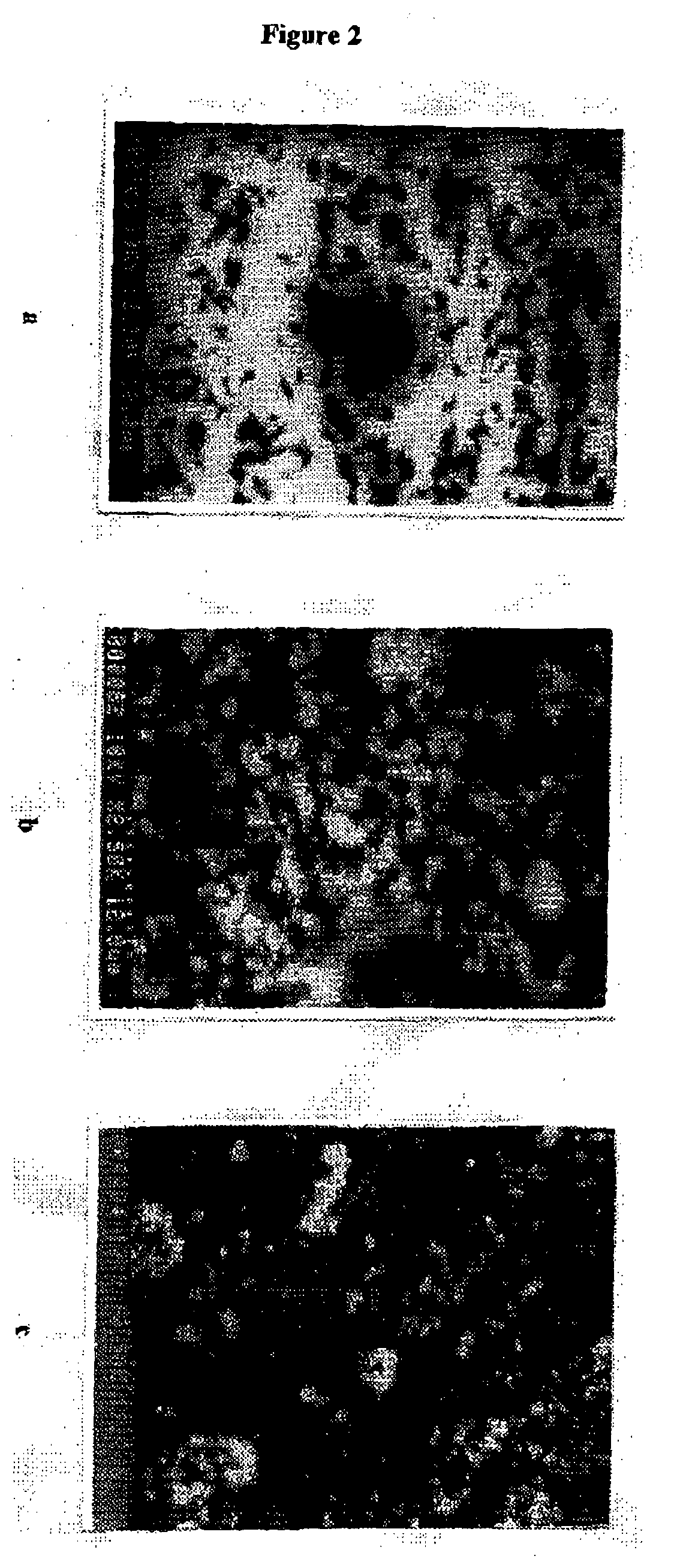
[0043] The H-acid (10.sup.-4M) solution of5 L is continuously run through the reactor at the flow rate of 750 ml min.sup.-1 using circulation pump. The degradation experiments were monitored under solar illumination. A partial view of the thin film fixed bed photocatalytic reactor is given in FIG. 1. The following characterization of TiO.sub.2 thin films were used. Scanning electron micrographs (SEM): Thin films of TiO.sub.2 prepared by spray technique were analyzed by SEM. FIG. 2 shows the scanning electron micrographs of binder bound TiO.sub.2 thin films a) binder film b) before use and c) after 30 days of use. From the micrographs it is observed that the TiO.sub.2 is present on the surface of the film and that the binder matrix does not cover it. The binder matrix improves the adherence of the photocatalyst that is necessary to obtain a good photocatalytic activity. TiO.sub.2 (white spots in FIG. 2b and 2c) is still present on the films even after continuous use for 30 days. The fil
example 2
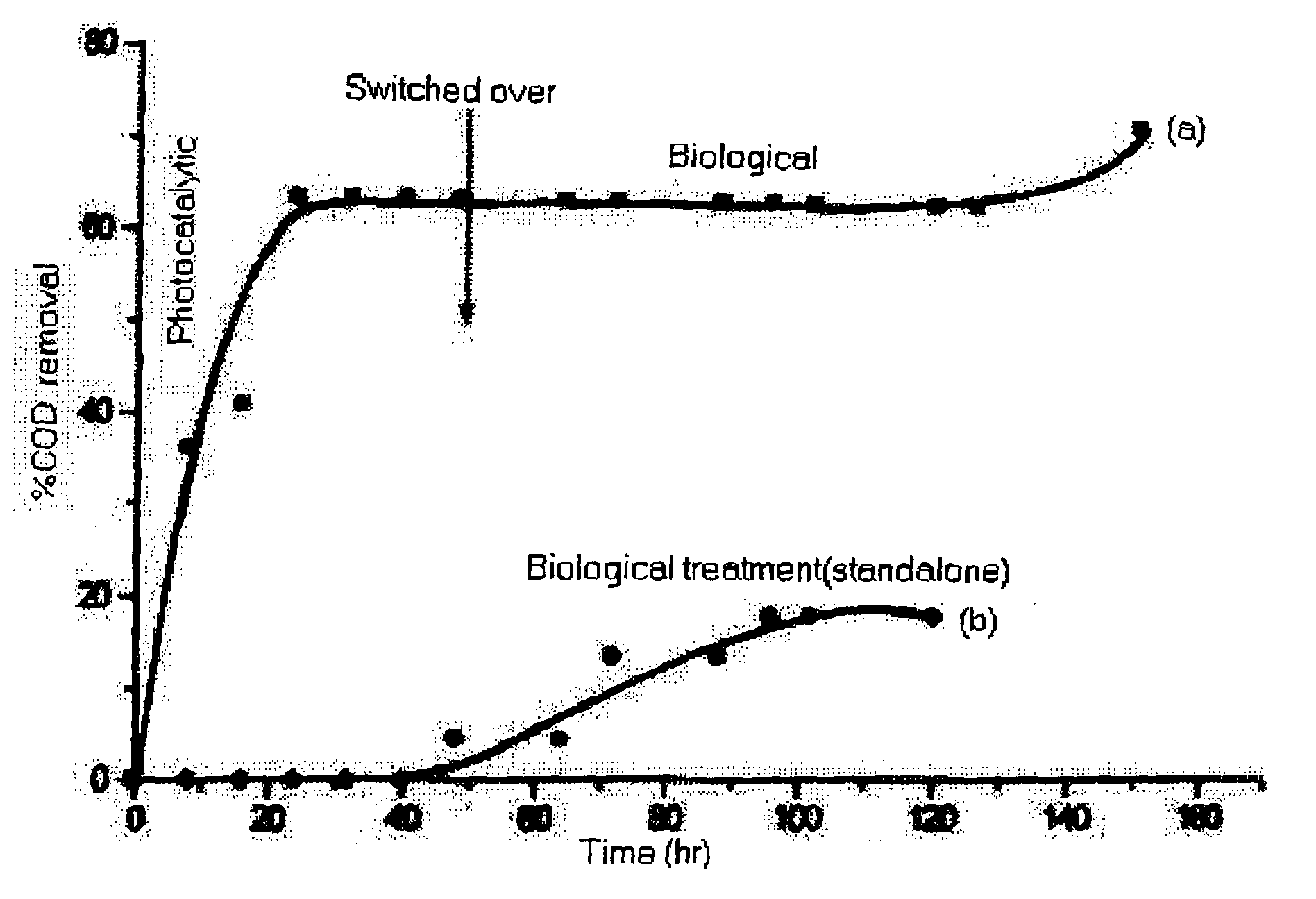
[0044] Degradation of H-acid on immobilized TiO.sub.2 using TFFBR was monitored under solar illumination for 3 days. H-acid of 5 L solution was run through TFFBR of area 7488 cm.sup.2 at a flow rate of 750 ml min.sup.-1. To compensate concentration due to evaporation, water was added at regular intervals. Samples collected at end of experiment were analyzed for COD and BOD values in order to evaluate the extent of biodegradability of H-acid. COD removal was 62% and there was an increase of BOD at the end of 3.sup.rd day of solar photocatalytic experiments as shown in Table 3. Also the data on common industrial effluent treatment data containing the COD reductions using slurry and thin film fixed bed reactor are given in FIG. 4.
3TABLE 3 Photocatalytic degradation of H-acid over TiO.sub.2 suspension (using UV light) and TiO.sub.2 thin film over Cuddapah stone (using Solar illumination). TiO.sub.2 Suspension TiO.sub.2 Film. Irradiation time: Parameters Irradiation time: 5 h (%) 15 h (3 da
example 3
[0045] In order to check the role of binder and its interaction with the film if any, the Diffuse Reflectance Spectra of TiO.sub.2 films were recorded. The DRS of the films shows no shift in the band gap of the TiO.sub.2 after immobilization (FIG. 3), Thus the TiO.sub.2 does not interact with the binder which is just used as an adhesive for holding the catalyst intact and enhancing the strength of the film. In the method developed for the immobilization of TiO.sub.2 the adherence of TiO.sub.2 improved by using the binder. On drying, the binder binds TiO.sub.2 to the support surface and forms a matrix like network that maintains TiO.sub.2 particles without affecting their photocatalytic activity. The TFFBR was used continuously in order to test the strength of the film and it was noted at the end of 30 days of treatment, the film was intact and the activity of TiO.sub.2 remained almost unchanged. The film was then analyzed by SEM and DRS. The presence of TiO.sub.2 on the surface of bind
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap