Long lasting inhibitors of viral infection
a technology of inhibitors and viral infections, applied in the direction of biocide, drug compositions, peptide/protein ingredients, etc., can solve the problems of short plasma half-life of peptides in vivo, and achieve the effect of increasing the stability in vivo of modified peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
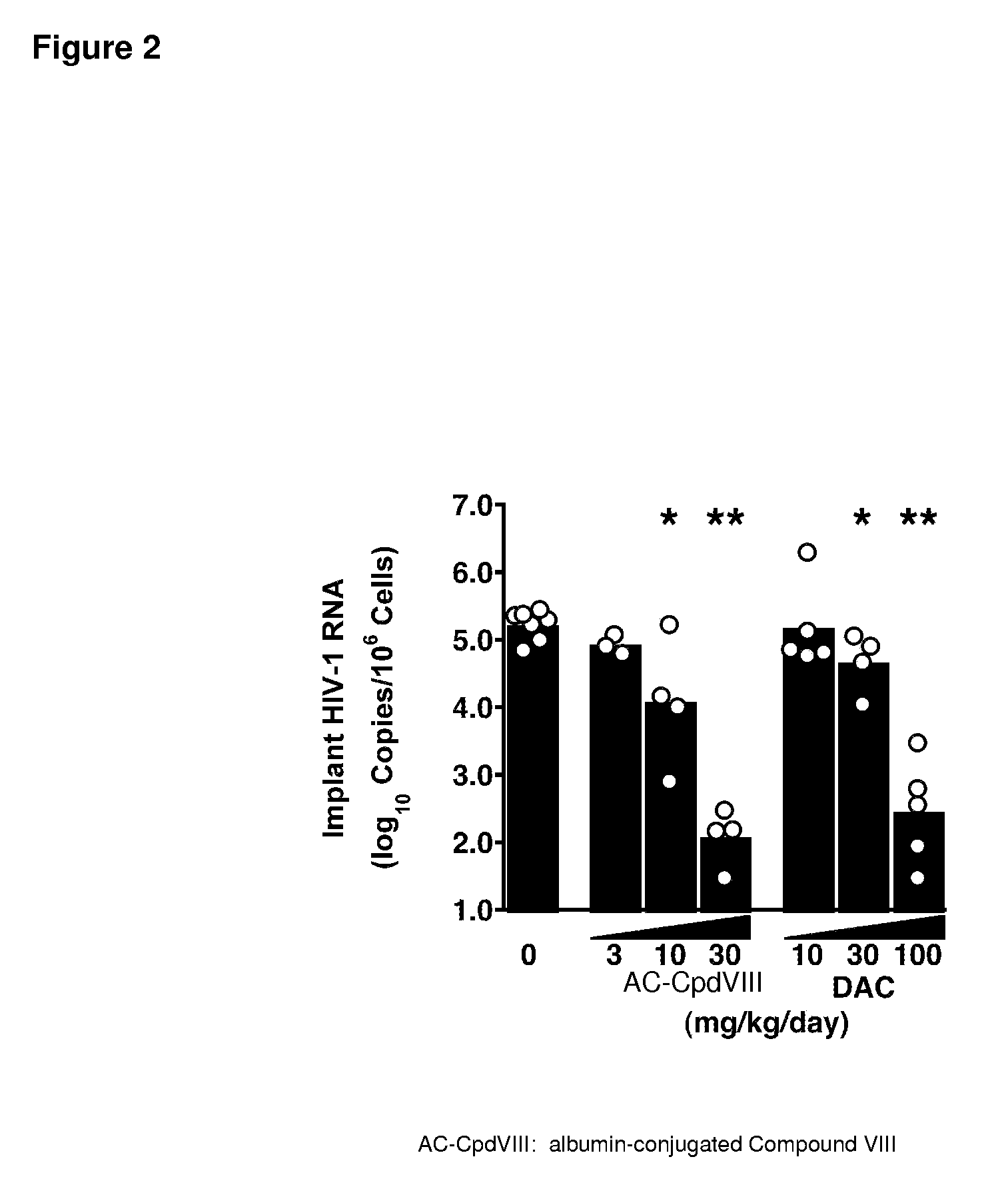
Comparison of the Antiviral Activity of a Single Pre-Exposure Dose of Albumin-Conjugated Compound VIII, and Truvada™ Against HIV-1 NL4-3 in SCID-hu Thy / Liv Mice
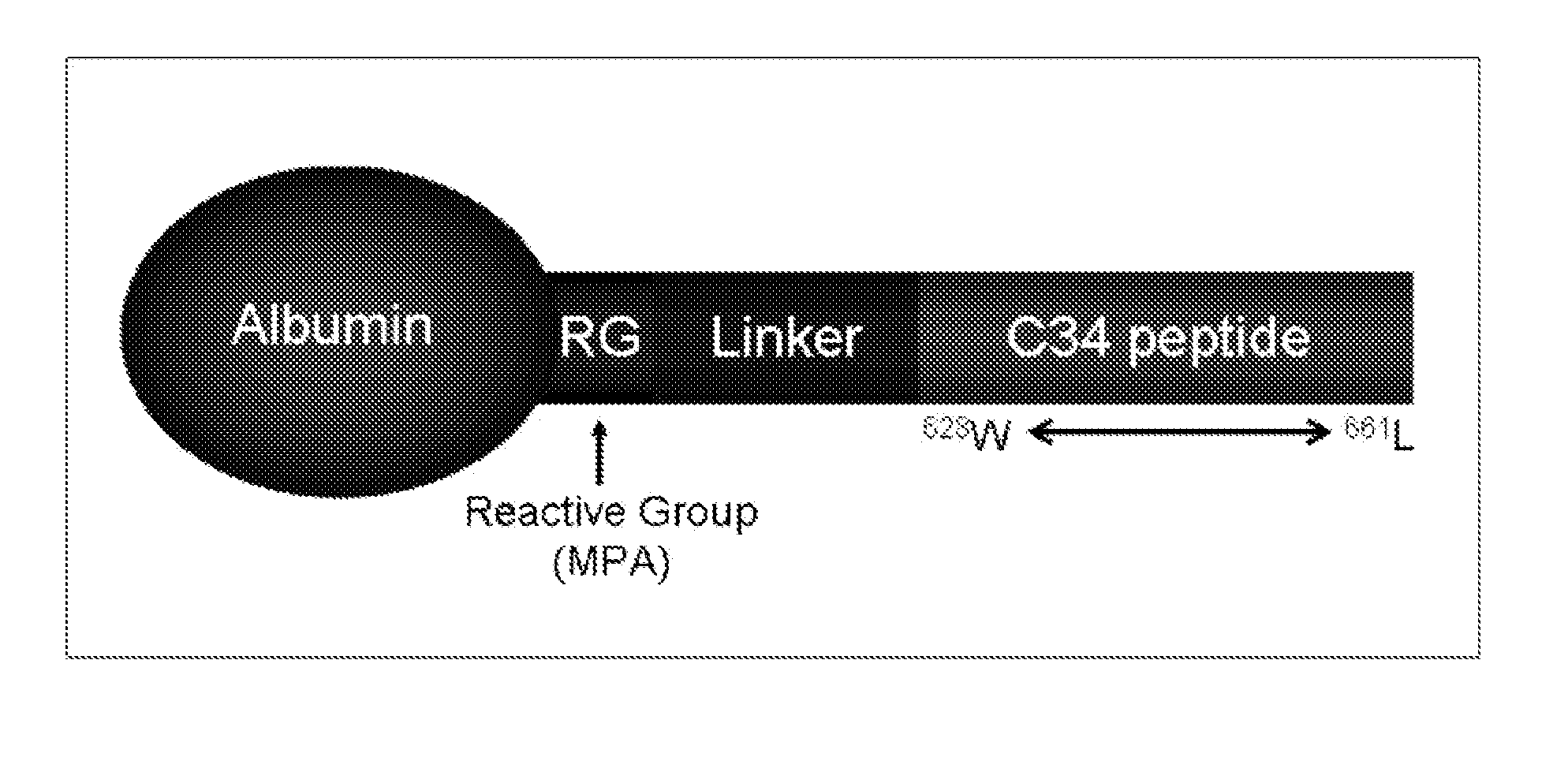
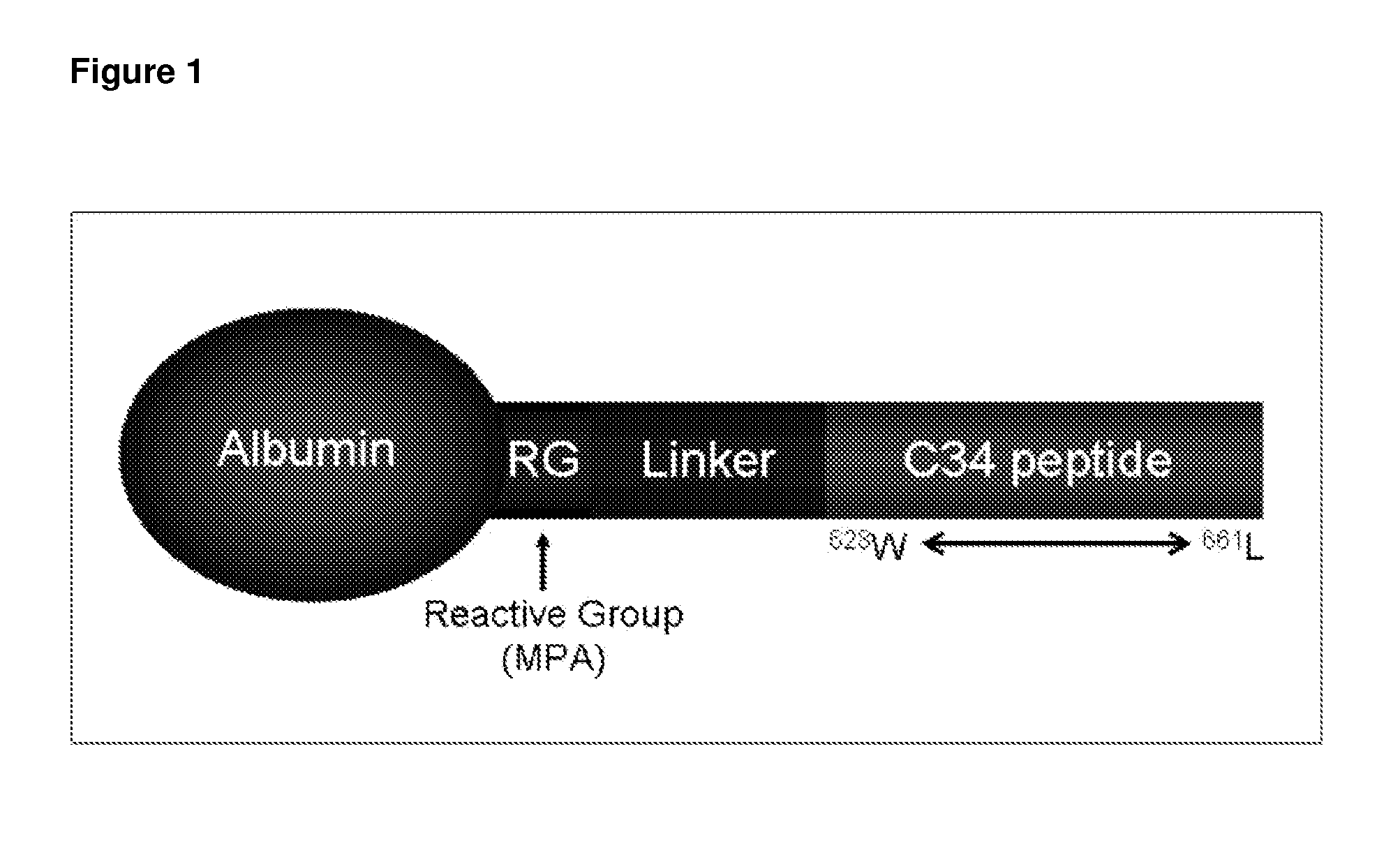
[0199]Albumin-conjugated Compound VIII, an albumin-conjugated peptide fusion inhibitor was modeled on the fusion inhibitor C34 (C34). It was designed to facilitate less frequent dosing in humans by increasing in vivo half-life (>10 days) and sustaining plasma levels compared with unconjugated peptide. The drug is a 1:1 covalent conjugate with specific attachment of the fusion-inhibiting peptide on cysteine 34 of albumin. The peptide is predicted to bind to the N-heptad repeat of gp41. The activity of a single pre-exposure dose of albumin-conjugated Compound VIII, was evaluated in SCID-hu Thy / Liv mice infected with HIV-1 NL4-3 24 hours after subcutaneous administration of one large dose of the C34 derivative of the present invention albumin-conjugated Compound VIII (60 or 200 mg / kg calculated amount for Compound VIII peptid
example 2
Evaluation of the Antiviral Activity of Albumin-Conjugated Compound Viii with Decreasing Dosing Frequency and Delayed Dosing Against HIV-1 NL4-3 in SCID-hu Thy / Liv Mice Treated by Subcutaneous Injection
[0203]
TABLE 4ProtocolImplantation dateMay 18, 2006Implant age18 weeksDonor ID#051706Inoculation dateSep. 19, 2006VirusHIV-1 NL4-3; batch lipo IV (diluted 1:3)Inoculum1,000 TCID50 per implantTermination dateOct. 10, 2006 (21 days after inoculation)Drugsalbumin-conjugated Compound VIII), (Conjuchem,Montreal, lots #7 / 13 / 06 and #9 / 28 / 06)VehicleN / ARoutesubcutaneousDosingtwice daily, or every fourth day, or every eighthday for 22 daysVolume200 μl per dose (400 μl foronce-daily dosing)Treatment initiation1 day before, 1 day, or 5 days after virus inoculation
TABLE 5ResultsHIV-1RNAlog10FACS analysisp24copies / Gag-p24+Mice / DoseDosing(pg / 106(% of106thymocytesCD4+CD8+GroupgroupVirusDrug(mg / kg / day)Frequencycells)control)cells(%)(%)A7NL4-3albumin-20 (300Q4D 8.5 ± 4.8* 1.6 ± 0.911.
example 3
Example 3A
Experimental Procedures
[0204]The following procedures were used throughout the experiments performed to obtain the results discussed in detail below.
[0205]Peptide Synthesis
[0206]Synthesis of the CHR peptide analogs were performed using an automated solid-phase procedure on a Symphony Peptide Synthesizer with manual intervention during the generation of the peptides. The synthesis was performed on Fmoc-protected Ramage amide linker resin, using Fmoc-protected amino acids. Coupling was achieved by using O-benzotriazol-1-yl-N,N,N′,N′-tetramethyl-uronium hexafluorophosphate (HBTU) and diisopropylethylamine (DIEA) as the activator cocktail in N,N-dimethylformamide (DMF) solution. The Fmoc protective group was removed using 20% piperidine / DMF. A Boc-protected amino acid was used at the N-terminus in order to generate the free α-N-terminus following cleavage of the peptides from the resin. Sigmacoated glass reaction vessels were used during the synthesis.
[0207]When the maleimido i
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap