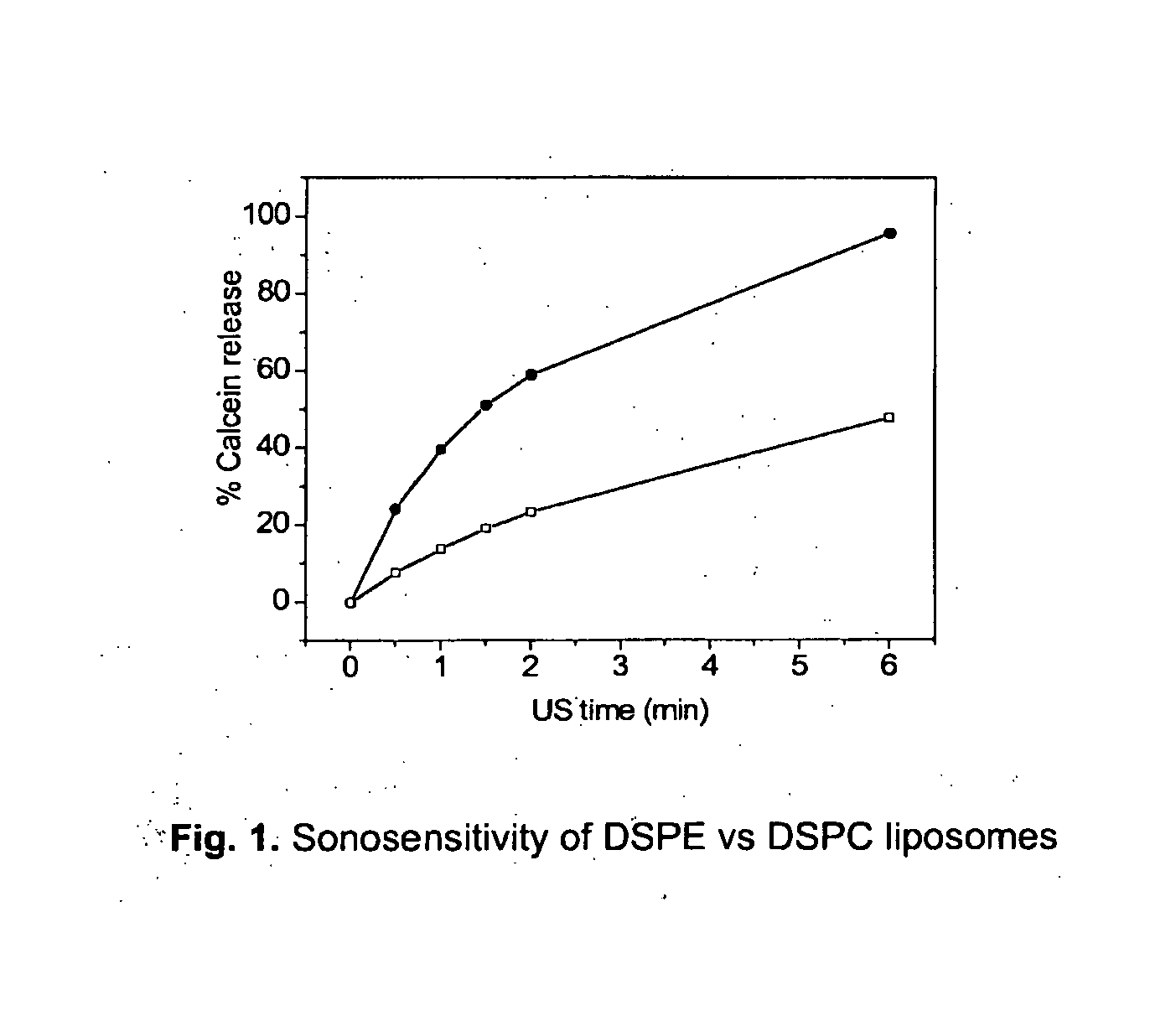
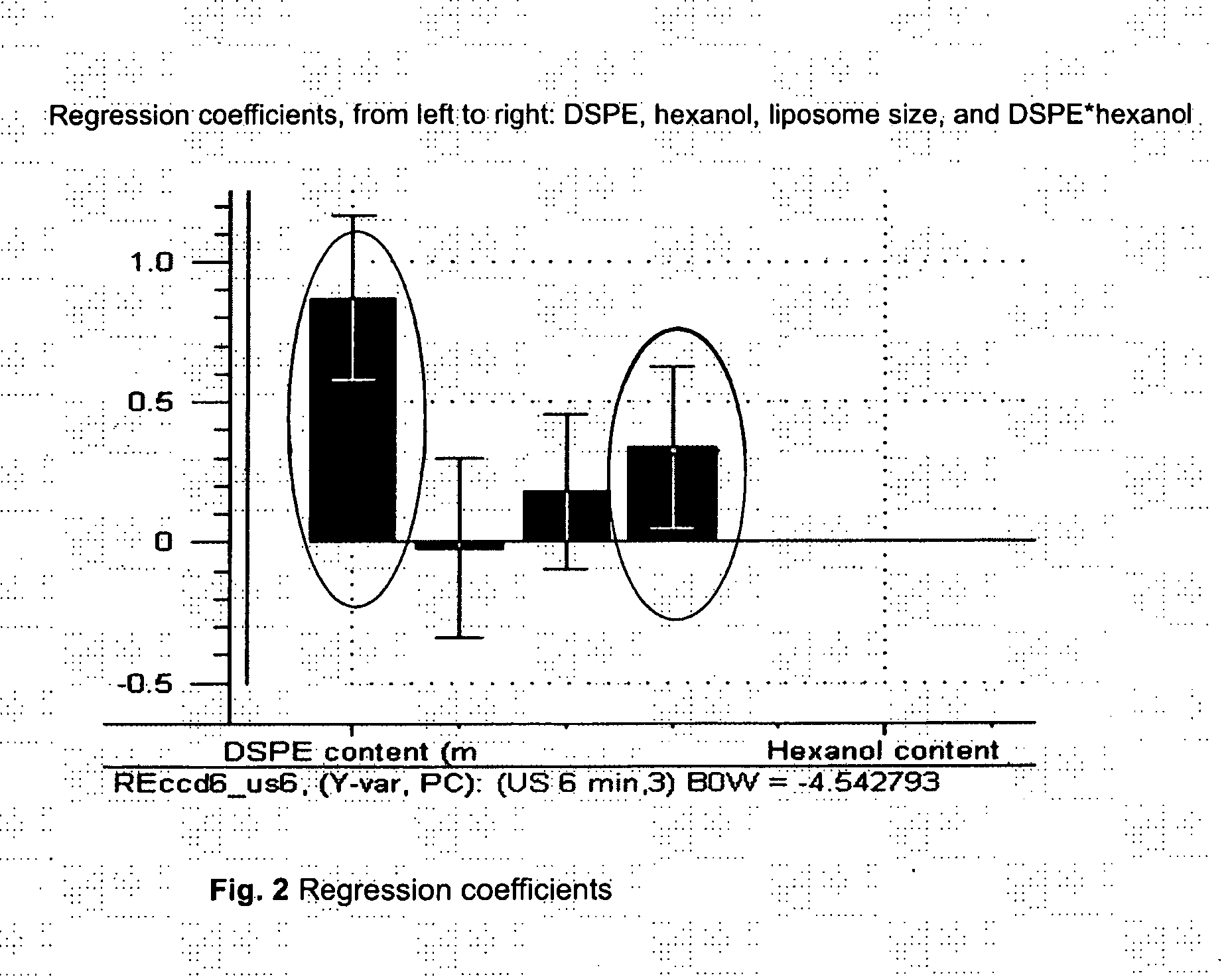
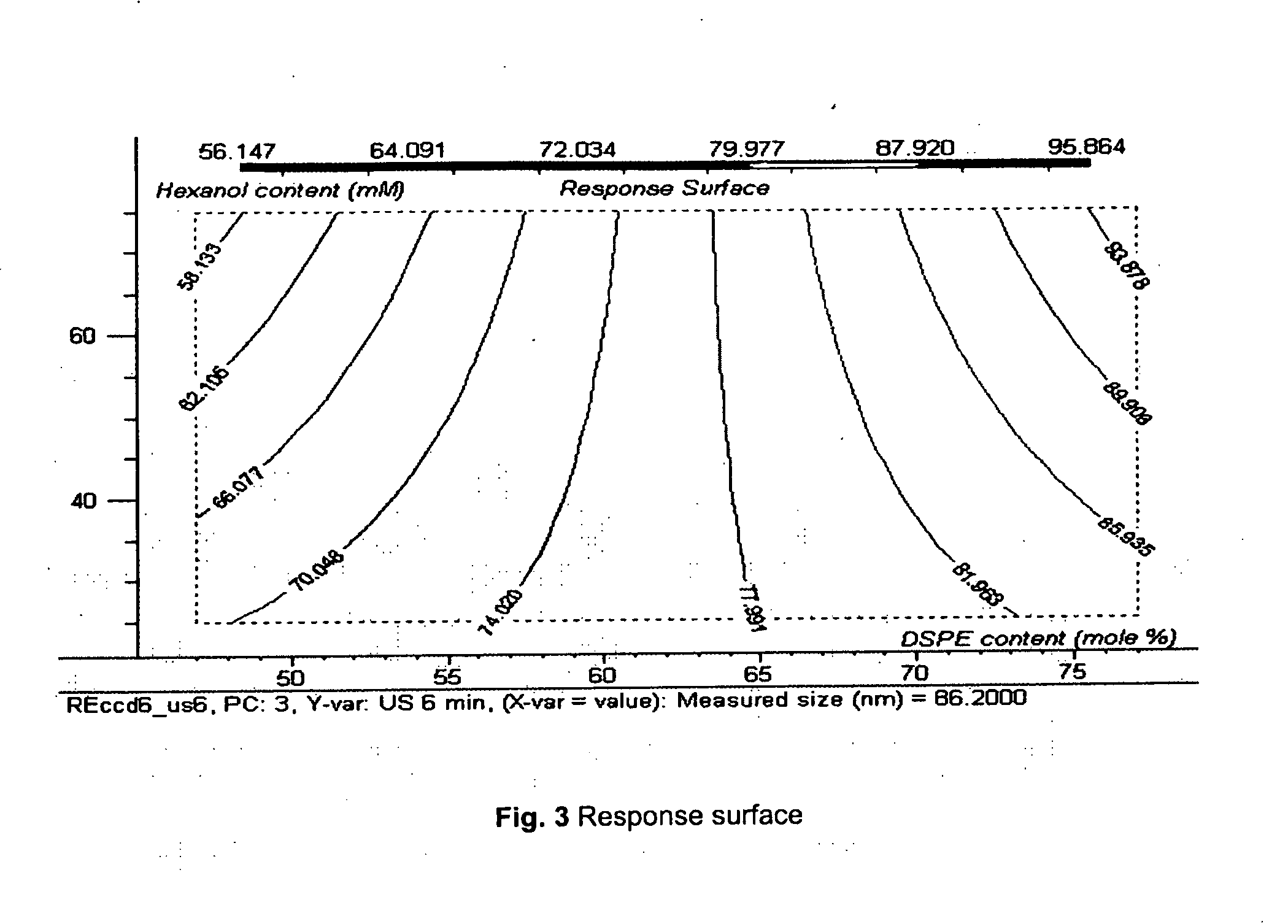
Acoustically sensitive drug delivery particles comprising low concentrations of phosphatidylethanolamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Liposomes Containing Fluorescent Drug Marker Calcein
[0084]DSPC, DSPE, DOPE, and DSPE-PEG 2000 were purchased from Genzyme Pharmaceuticals (Liestal, Switzerland). Cholesterol, calcein, HEPES, TRITON-X100 (10% solution), sodium azide and sucrose were obtained from Sigma Aldrich. Hexanol was supplied by BDH Chemicals Ltd. (Poole, England).
[0085]Calcein carrying liposomes (liposomal calcein) of different membrane composition were prepared using the thin film hydration method (Lasic 1993). The nominal lipid concentration was 16 mg / ml. Liposomes were loaded with calcein via passive loading, the method being well known within the art. The hydration liquid consisted of 10 mM HEPES (pH 7.4) and 50 mM calcein. For the preparation of liposomal calcein containing hexanol, the hydration liquid was supplemented with a given amount of hexanol 2 days prior to usage in the lipid film hydration step.
[0086]After three freeze-thaw cycles, the liposomes were down-sized to 80-90 nm by extru
example 2
Characterisation of Calcein Containing Liposomes
[0088]Liposomes were characterised with respect to key physicochemical properties like particle size, pH and osmolality by use of well-established methodology.
[0089]The average particle size (intensity weighted) and size distribution were determined by photon correlation spectroscopy (PCS) at a scattering angle of 173° and 25 deg C. (Nanosizer, Malvern Instruments, Malvern, UK). The width of the size distribution is defined by the polydispersity index. Prior to sample measurements the instruments was tested by running a latex standard (60 nm). For the PCS measurements, 10 μL of liposome dispersion was diluted with 2 mL sterile filtered isosmotic sucrose solution containing 10 mM HEPES (pH 7.4) and 0.02% (w / v) sodium azide. Duplicates were analysed.
[0090]Osmolality was determined on non-diluted liposome dispersions by freezing point depression analysis (Fiske 210 Osmometer, Advanced Instruments, MA, US). Prior to sample measurements, a r
example 3
US Mediated Release Methodology and Quantification for Calcein Containing Liposomes
[0091]Liposome samples were exposed to 20 or 40 kHz ultrasound up to 6 min in a custom built sample chamber as disclosed in Huang and MacDonald (Huang and Macdonald 2004). The US power supply and converter system was one of two systems: (1) ‘Vibra-Cell’ ultrasonic processor, VC 750, 20 kHz unit with a 6.35 cm diameter transducer or (2) ‘Vibra-Cell’ ultrasonic processor, VC754, 40 kHz unit with a 19 mm cup horn probe, both purchased from Sonics and Materials, Inc. (USA). Pressure measurements were conducted with a Bruel and Kjaer hydrophone type 8103.
[0092]Both systems were run at the lowest possible amplitude, i.e. 20 to 21% of maximum amplitude. At this minimal amplitude acoustic pressure measurements in the sample chamber gave=430 kPa (pk-pk) for 20 kHz and =240 kPa (pk-pk) for 40 kHz.
[0093]For the US measurements, liposome dispersions were diluted in a 1:500 volume ratio, with isosmotic sucrose soluti
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap