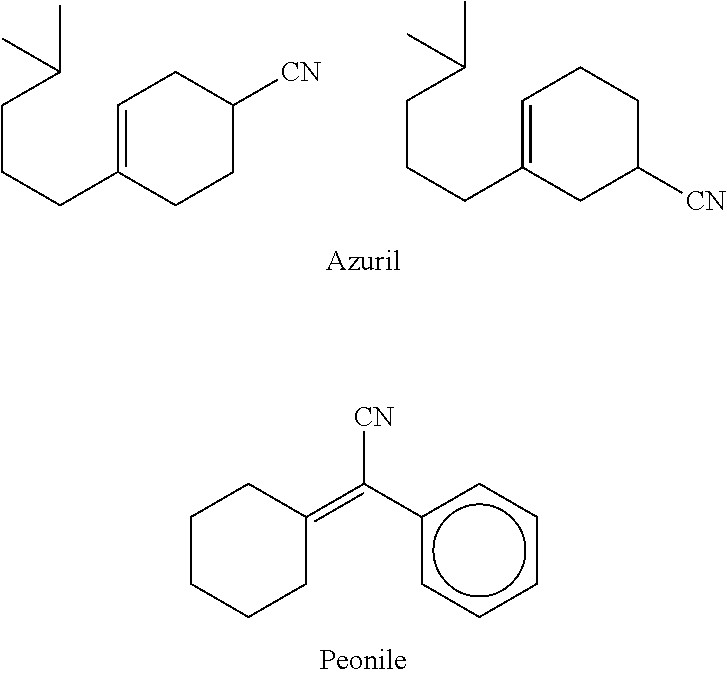
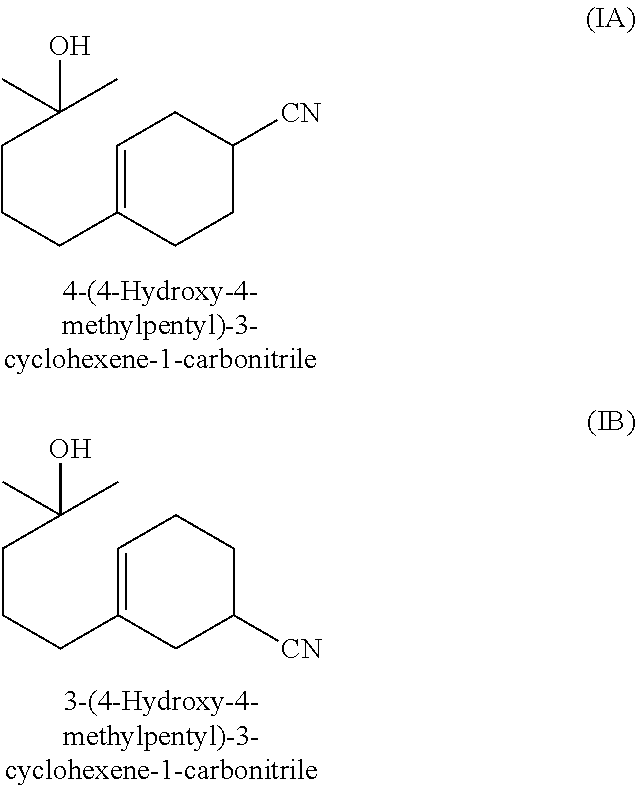
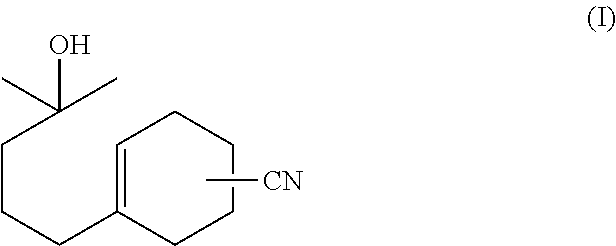
4(3)-(4-hydroxy-4-methylpentyl)-3-cyclohexene-1-carbonitrile
a technology of cyclohexene and cyclohexene, which is applied in the direction of perfume formulations, detergent compounding agents, organic chemistry, etc., can solve the problems of difficult to predict the change of fragrance notes and difficult to predict the harmonicity with other fragrance materials, and achieve good fragrance retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Production of 4(3)-(4-hydroxy-4-methylpentyl)-3-cyclohexene-1-carboxaldehyde oxime
[0139]
[0140]In a 200 mL flask, 32 g of 4(3)-(4-hydroxy-4-methylpentyl)-3-cyclohexene-1-carboxaldehyde (Lyral, Trade Name of IFF, 0.15 mole), 30 g of isopropyl alcohol, 19 g of hydroxylamine sulfate (0.11 mole, 0.73 times by mole with respect to aldehyde and 1.46 times by mole in terms of hydroxylamine), and 35 g of ion exchanged water were added sequentially. This was heated to 45° C. while being stirred in a nitrogen atmosphere. While the reaction temperature was kept at 40 to 50° C., 33 g (0.25 mole) of 30 mass % sodium hydroxide aqueous solution was dropped over 30 minutes. Further, heating and stirring were continued for one hour. After the reaction solution was cooled to room temperature, an aqueous layer was extracted by settled separation. An organic layer was washed with a 10 mass % sodium sulfate aqueous solution and then isopropyl alcohol was distilled, which yielded 39 g of a pale yellow liquid
example 1
Production of 4(3)-(4-hydroxy-4-methylpentyl)-3-cyclohexene-1-carbonitrile
[0152]
[0153]In a 300 mL flask with a Dean-Stark dehydration tube attached thereto, 35 g of 4(3)-(4-hydroxy-4-methylpentyl)-3-cyclohexene-1-carboxaldehyde oxime (the crude product of Production Example 1, with a pure content of 26 g, 0.12 mole), 3.0 g of powdered sodium hydroxide (75 mmoles, 8.6 mass % with respect to the oxime intermediate), and 100 g of toluene were placed, which then was refluxed continuously for three hours until water distillate stopped coming out. After the reactant was cooled to room temperature, 50 g of water was added thereto to dissolve sodium hydroxide, which then was further neutralized with acetic acid. An aqueous layer was extracted by settled separation. Further, an organic layer was washed twice with a 10 mass % sodium sulfate aqueous solution and then toluene was distilled, which yielded 33 g of an orange liquid product. As a result of gas chromatography quantitative analysis of t
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap