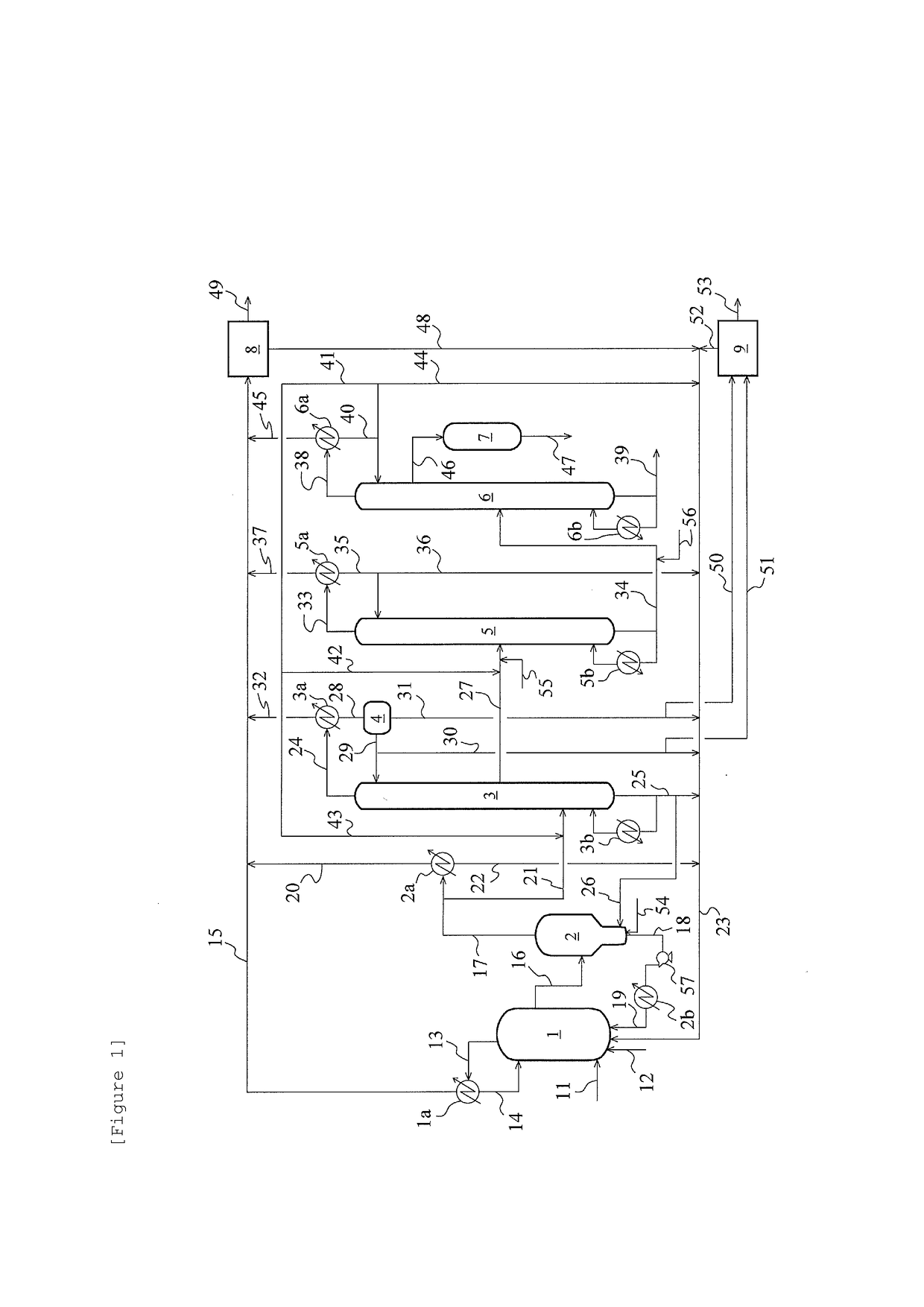
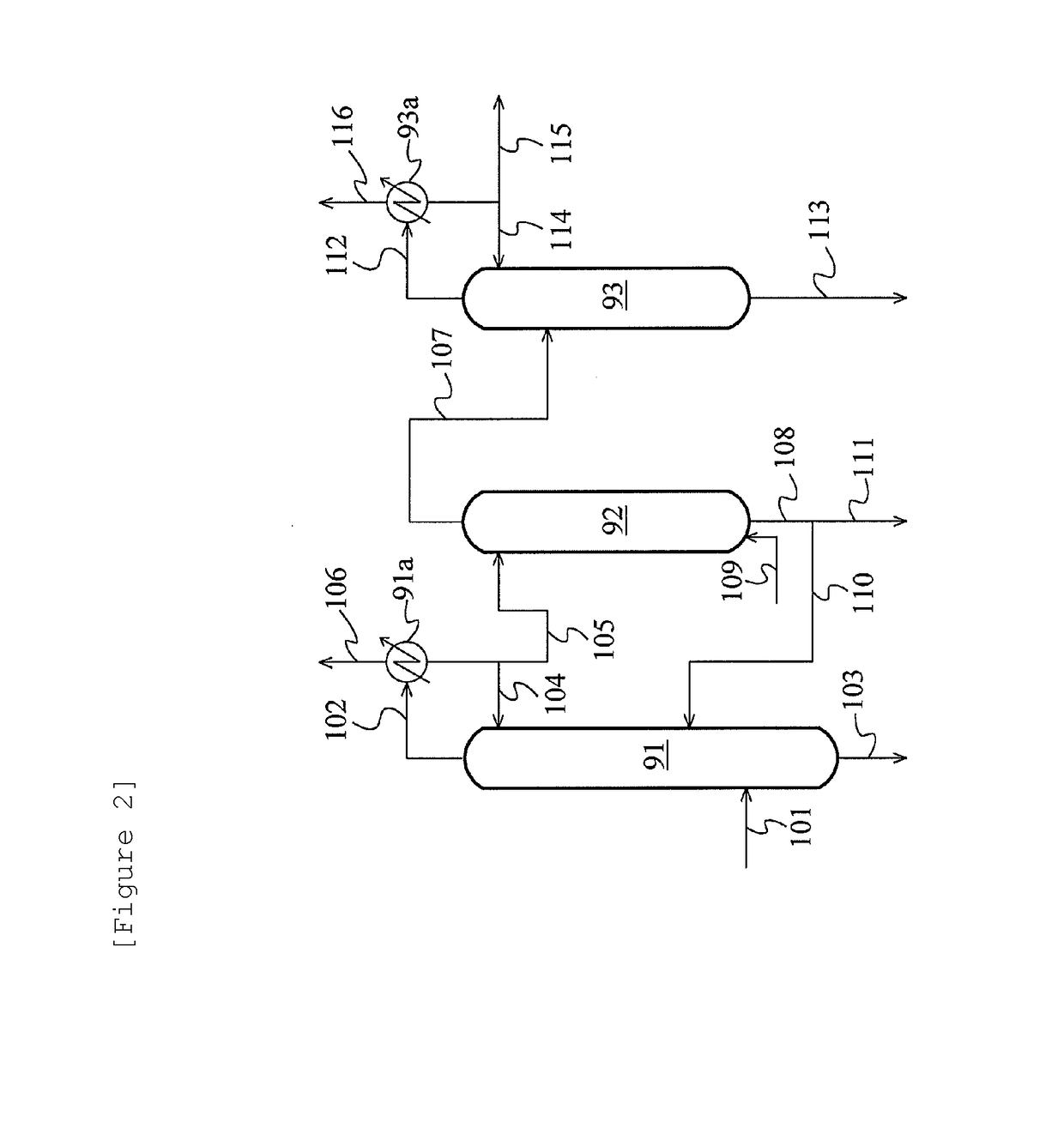
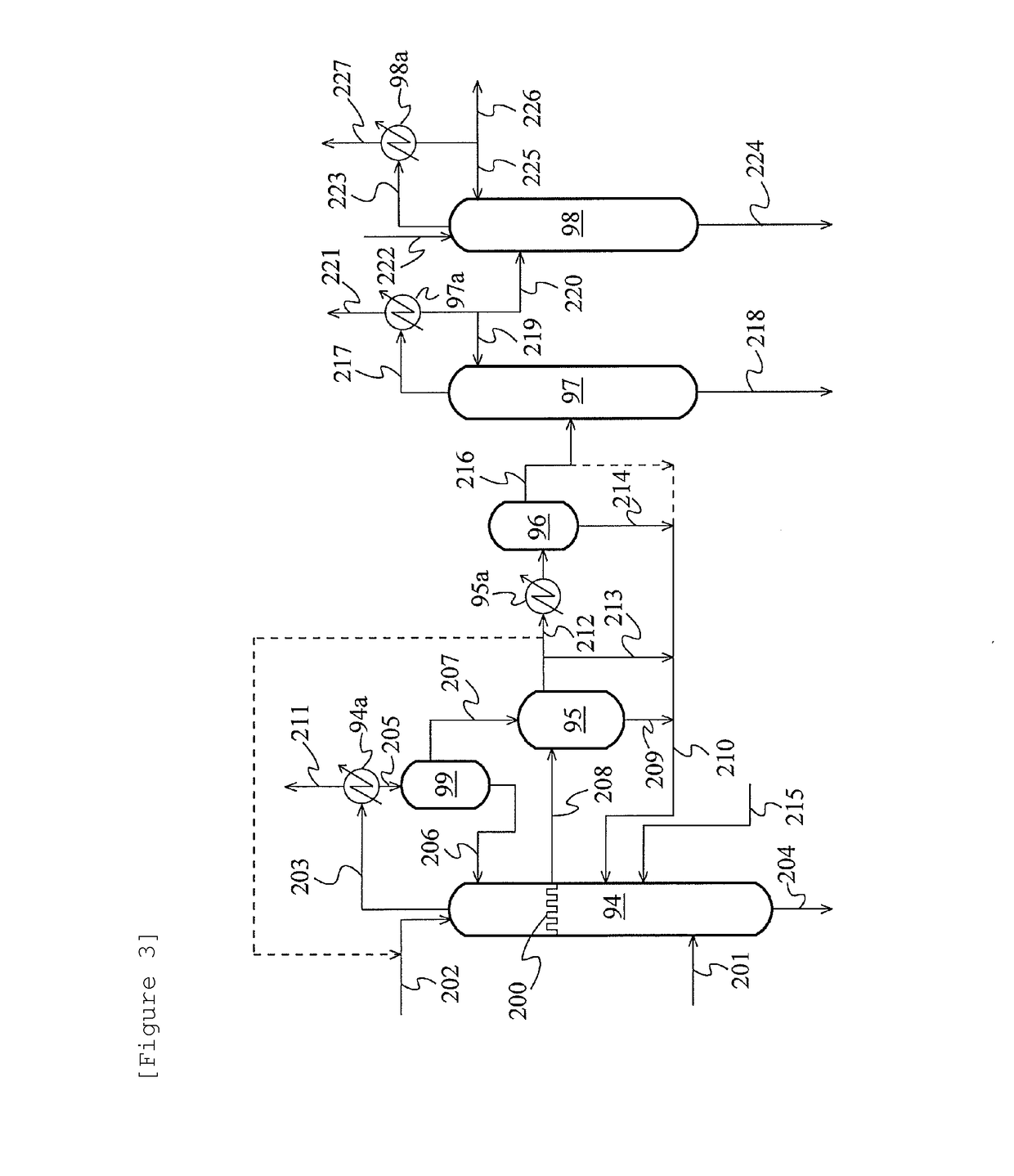
Method for producing acetic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Comparative Example 1
[0092]459 g of propionic acid and 46 g of acetic acid were placed in a 1000 ml zirconium autoclave, and a test piece (size: 36 mm×25 mm×2.5 mm) of zirconium (Zr), a nickel-based alloy Hastelloy B2 (manufactured by Oda Koki Co., Ltd., “HB2”), a nickel-based alloy Hastelloy C (manufactured by Oda Koki Co., Ltd., “HC276”), or stainless steel SUS316 (manufactured by Umetoku Inc., “SUS316”) as each material was placed in the autoclave, which was then covered with a lid. The liquid in the autoclave was bubbled with nitrogen to purge oxygen dissolved in the liquid. Then, the operation of increasing the atmospheric pressure with nitrogen to 1 MPaG, which was then reduced to the atmospheric pressure was carried out three times. Then, nitrogen gas was introduced thereto to 4 MPaG, and the pressure was discharged until atmospheric pressure. Then, the autoclave was heated in an oil bath such that the liquid temperature in the autoclave was 165° C. The static pressure after the
Example
Comparative Example 2
[0093]A corrosion test was conducted in the same way as in Comparative Example 1 except that the feed composition was changed to 100% of acetic acid. The feed composition, conditions for the corrosion test, and results thereof are shown in Tables 1 and 2.
Example
Examples 1 to 15
[0094]A corrosion test was conducted in the same way as in Comparative Example 1 except that the feed composition and the temperature were changed. The feed composition, conditions for the corrosion test, and results thereof are shown in Tables 1 and 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap