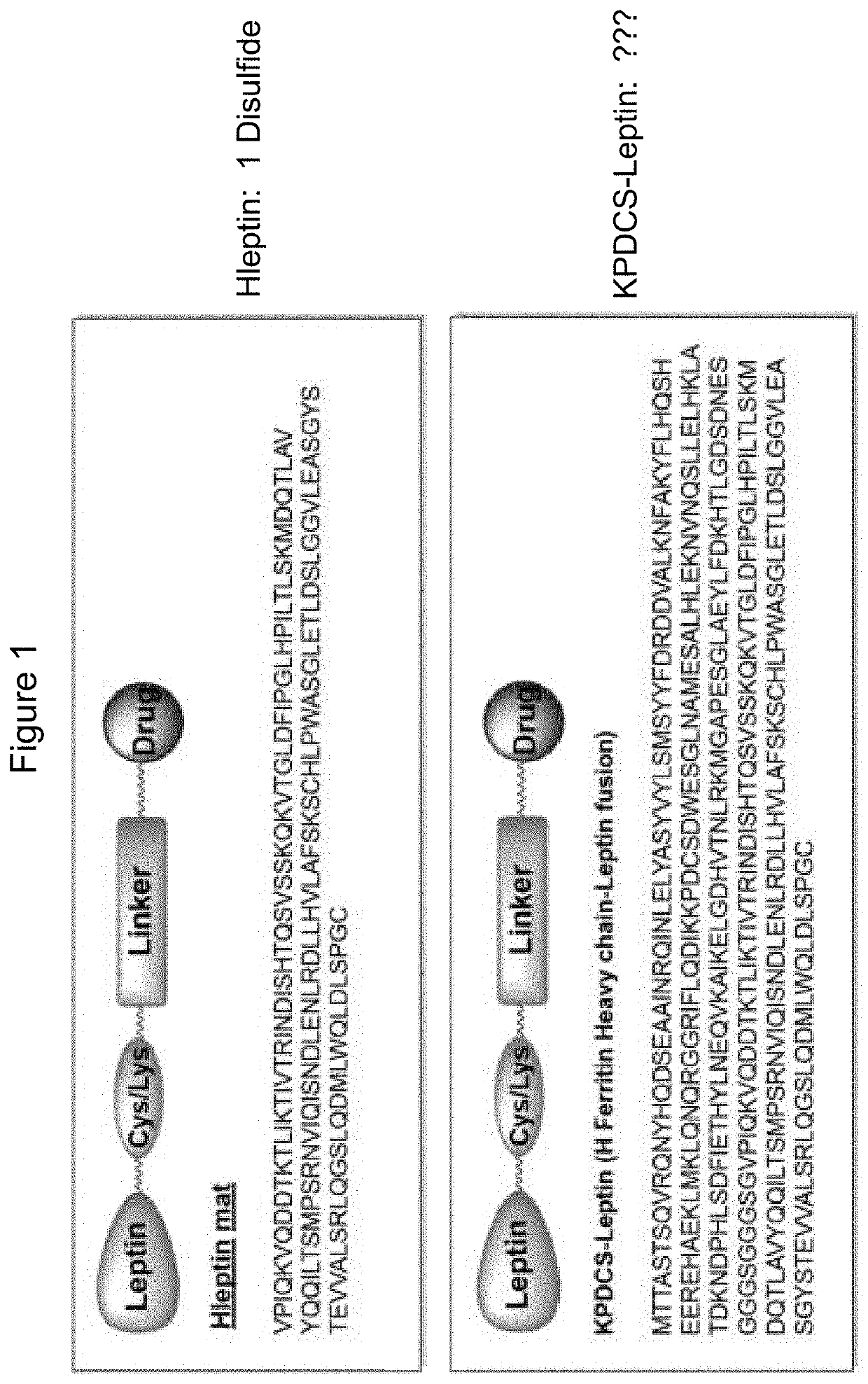
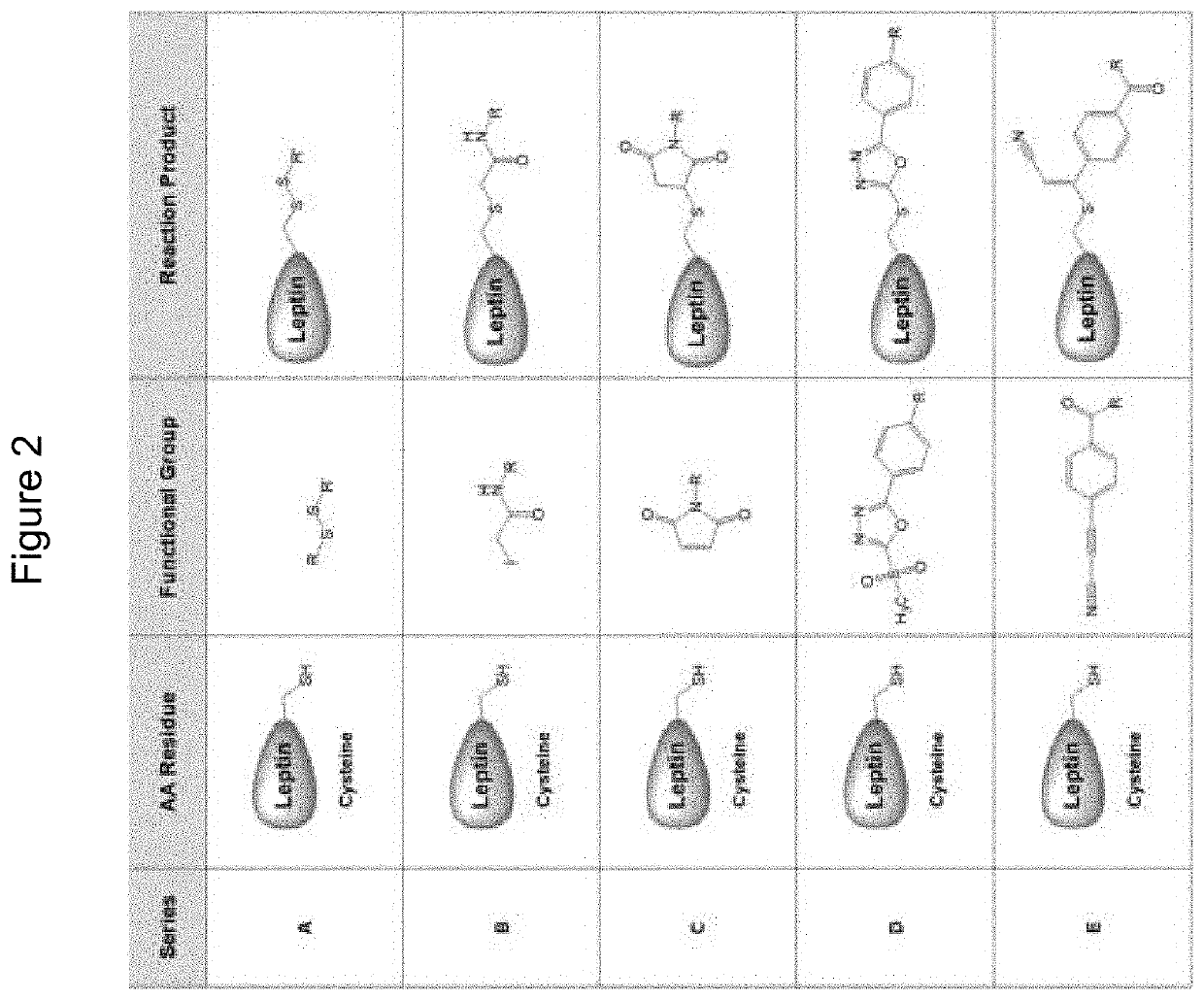
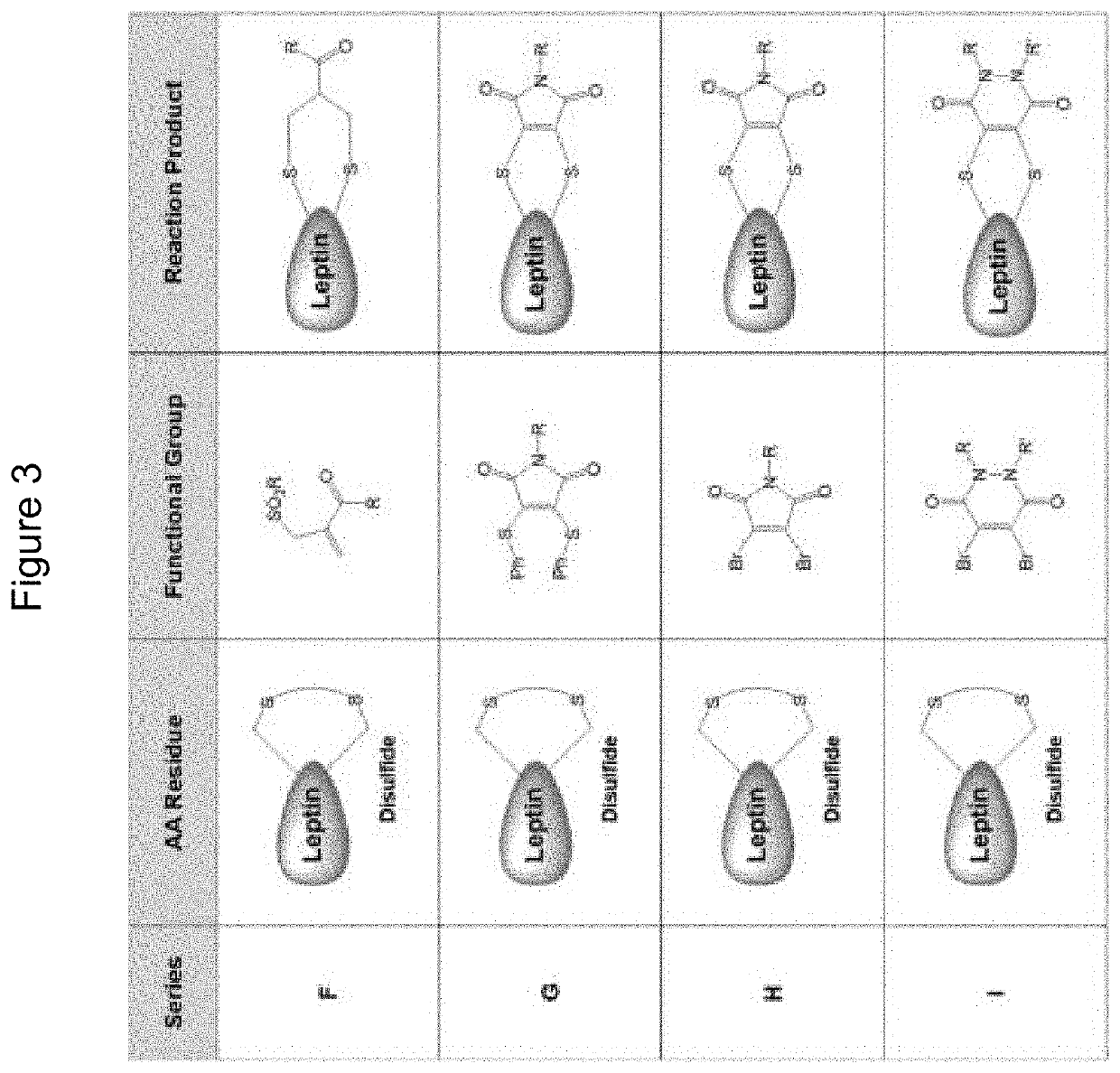
Compounds, compositions, methods, and uses for treating leptin resistance, obesity, diabetes mellitus and metabolic syndrome
a technology of leptin resistance and metabolic syndrome, applied in the field of chemical compounds, can solve the problems of insensitive exogenous leptin and high levels of circulating leptin in obese patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0274]Hypothetical examples are disclosed below to illustrate the invention. Persons skilled in the art will recognize many different variations of these hypotheticals based on the disclosure in this document and known prior art. All variations are intended to be within the scope of this invention. Therefore, the examples disclosed are not intended to limit the scope of the claims. Animal Distribution into Treatment Groups: Mice were distributed into treatment groups by body weight so that there is no significant difference between the means across groups.
[0275]Intraperitoneal (IP) Dosing: A 27½″ gauge needle is used for injection. Injection volume for the study is 5 ml / kg per injection. Restrain the mouse and identify the injection location which is above or equal to the top of the hips and below the middle of the abdomen, generally just to the right of the midline.
[0276]Glucose Tolerance Test (ipGTT): Mice are weighed and transferred to individual clean cages that provide water and
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap